Ann Lab Med.
2014 Jan;34(1):43-50. 10.3343/alm.2014.34.1.43.
Effects of Platelet Lysate Preparations on the Proliferation of HaCaT Cells
- Affiliations
-
- 1Green Cross Laboratories, Yongin, Korea.
- 2Department of Laboratory Medicine, School of Medicine, Ajou University, Suwon, Korea. limyoung@ajou.ac.kr
- 3Department of Otolaryngology, School of Medicine, Ajou University, Suwon, Korea.
- KMID: 1781359
- DOI: http://doi.org/10.3343/alm.2014.34.1.43
Abstract
- BACKGROUND
Standard protocols are lacking for the preparation of platelet lysates (PL) as an alternative to using fetal bovine serum as a cell culture supplement. This study aimed to establish optimum conditions for preparing PL for use in cell cultures.
METHODS
Cell density in three pooled platelet concentrates (PC) were adjusted to 1x10(12)/L and 2x10(12)/L. PL was prepared from PC by 1 to 3 freeze-thaw (FT) cycles. HaCaT cells were cultured in media supplemented with 5% or 10% PL. Cell numbers were estimated using a Cell Counting Kit-8 (CCK-8; Dojindo Laboratories, Japan). Growth factors were quantified by using the Luminex 200 system (Luminex Corporation, USA).
RESULTS
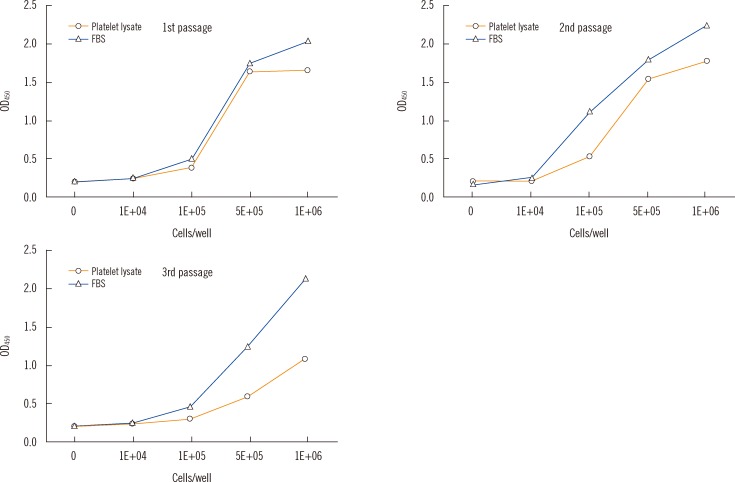
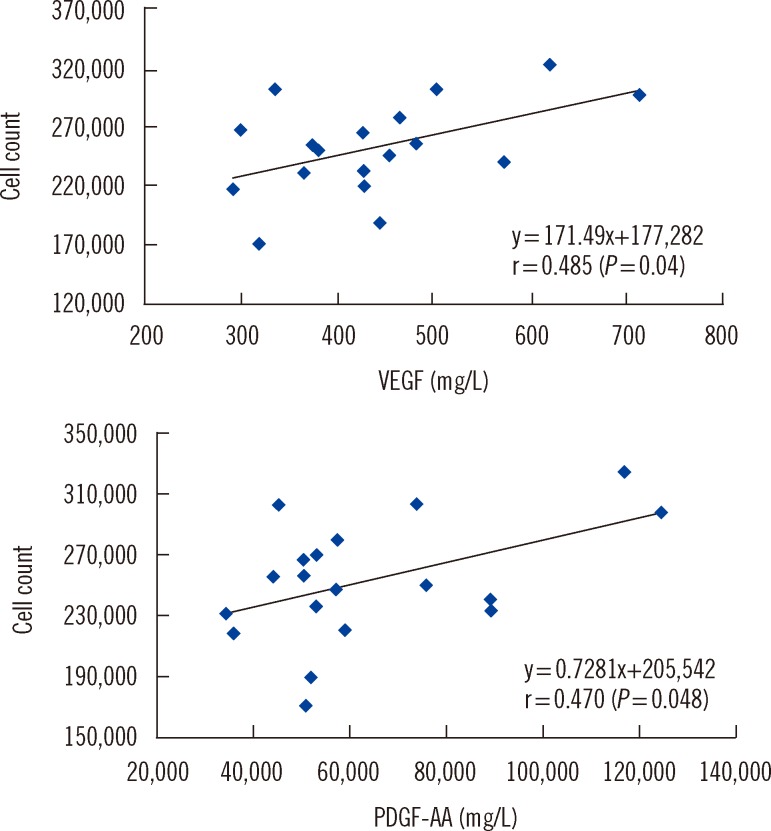
Cell proliferation rates in the presence of PLs were similar when prepared from PCs of both cell densities. The rates were higher in media containing 5% PL than 10% PL when prepared by two FT cycles. Concentrations of vascular endothelial growth factor (VEGF), platelet-derived growth factor-AB/BB (PDGF-AB/BB), PDGF-AA, and epidermal growth factor (EGF) were significantly higher in PL prepared from PC with a cell density of 2x10(12)/L than 1x10(12)/L PC. However, only VEGF and PDGF-AA concentrations in PLs were correlated with HaCaT cell counts.
CONCLUSIONS
The 5% PL from PC with a cell density of 1x10(12)/L prepared by two FT cycles treatment was the most effective condition that supported steady HaCaT cell proliferation. Our finding may be useful for preparing PL-supplemented cell culture media.
MeSH Terms
-
Blood Platelets/chemistry/*metabolism
Cell Line
Cell Proliferation/drug effects
Culture Media/pharmacology
Epidermal Growth Factor/chemistry/pharmacology
Humans
Platelet-Derived Growth Factor/chemistry/pharmacology
Vascular Endothelial Growth Factor A/chemistry/pharmacology
Culture Media
Epidermal Growth Factor
Platelet-Derived Growth Factor
Vascular Endothelial Growth Factor A
Figure
Cited by 1 articles
-
Human platelet lysate efficiency, stability, and optimal heparin concentration required in culture of mammalian cells
Hoda E. Mohamed, Mervat E. Asker, Nahla S. Kotb, Akram M. El Habab
Blood Res. 2020;55(1):35-43. doi: 10.5045/br.2020.55.1.35.
Reference
-
1. Tayapongsak P, O'Brien DA, Monteiro CB, Arceo-Diaz LY. Autologous fibrin adhesive in mandibular reconstruction with particulate cancellous bone and marrow. J Oral Maxillofac Surg. 1994; 52:161–165. PMID: 8295051.
Article2. Eppley BL, Pietrzak WS, Blanton M. Platelet-rich plasma: a review of biology and applications in plastic surgery. Plast Reconstr Surg. 2006; 118:147e–159e.
Article3. Mehta S, Watson JT. Platelet rich concentrate: basic science and current clinical applications. J Orthop Trauma. 2008; 22:432–438. PMID: 18594311.
Article4. Baeyens W, Glineur R, Evrard L. The use of platelet concentrates: platelet-rich plasma (PRP) and platelet-rich fibrin (PRF) in bone reconstruction prior to dental implant surgery. Rev Med Brux. 2010; 31:521–527. PMID: 21290856.5. Schallmoser K, Bartmann C, Rohde E, Reinisch A, Kashofer K, Stadelmeyer E, et al. Human platelet lysate can replace fetal bovine serum for clinical-scale expansion of functional mesenchymal stromal cells. Transfusion. 2007; 47:1436–1446. PMID: 17655588.
Article6. Bieback K, Hecker A, Kocaömer A, Lannert H, Schallmoser K, Strunk D, et al. Human alternatives to fetal bovine serum for the expansion of mesenchymal stromal cells from bone marrow. Stem Cells. 2009; 27:2331–2341. PMID: 19544413.
Article7. Reinisch A, Bartmann C, Rohde E, Schallmoser K, Bjelic-Radisic V, Lanzer G, et al. Humanized system to propagate cord blood-derived multi potent mesenchymal stromal cells for clinical application. Regen Med. 2007; 2:371–382. PMID: 17635045.8. Lange C, Cakiroglu F, Spiess AN, Cappallo-Obermann H, Dierlamm J, Zander AR. Accelerated and safe expansion of human mesenchymal stromal cells in animal serum-free medium for transplantation and regenerative medicine. J Cell Physiol. 2007; 213:18–26. PMID: 17458897.
Article9. Cho HS, Song IH, Park SY, Sung MC, Ahn MW, Song KE. Individual variation in growth factor concentrations in platelet-rich plasma and its influence on human mesenchymal stem cells. Korean J Lab Med. 2011; 31:212–218. PMID: 21779198.
Article10. Doucet C, Ernou I, Zhang Y, Llense JR, Begot L, Holy X, et al. Platelet lysates promote mesenchymal stem cell expansion: a safety substitute for animal serum in cell-based therapy applications. J Cell Physiol. 2005; 205:228–236. PMID: 15887229.
Article11. Schallmoser K, Strunk D. Preparation of pooled human platelet lysate (pHPL) as an efficient supplement for animal serum-free human stem cell cultures. J Vis Exp. 2009; 32:1523. PMID: 19881465.
Article12. Sundin M, Ringdén O, Sundberg B, Nava S, Götherström C, Le Blanc K. No alloantibodies against mesenchymal stromal cells, but presence of anti-fetal calf serum antibodies, after transplantation in allogeneic hematopoietic stem cell recipients. Haematologica. 2007; 92:1208–1215. PMID: 17666368.
Article13. Kakudo N, Minakata T, Mitsui T, Kushida S, Notodihardjo FZ, Kusumoto K. Proliferation-promoting effect of platelet-rich plasma on human adipose-derived stem cells and human dermal fibroblasts. Plast Reconstr Surg. 2008; 122:1352–1360. PMID: 18971718.
Article14. Valeri CR, Saleem B, Ragno G. Release of platelet-derived growth factors and proliferation of fibroblasts in the releasates from platelets stored in the liquid state at 22 degrees C after stimulation with agonists. Transfusion. 2006; 46:225–229. PMID: 16441599.15. García-Martínez O, Reyes-Botella C, Díaz-Rodríguez L, De Luna-Bertos E, Ramos-Torrecillas J, Vallecillo-Capilla MF, et al. Effect of platelet-rich plasma on growth and antigenic profile of human osteoblasts and its clinical impact. J Oral Maxillofac Surg. 2012; 70:1558–1564. PMID: 21864971.
Article16. Pawitan JA. Platelet rich plasma in xeno-free stem cell culture: the impact of platelet count and processing method. Curr Stem Cell Res Ther. 2012; 7:329–335. PMID: 22849700.17. Lim YA, Baik SY, Lee WG. The effect of freezing-thawing activated platelet rich plasmas (PRP) on the proliferations of bacteria. Korean J Blood Transfus. 2011; 22:221–230.18. Mazzocca AD, McCarthy MB, Chowaniec DM, Dugdale EM, Hansen D, Cote MP, et al. The positive effects of different platelet-rich plasma methods on human muscle, bone, and tendon cells. Am J Sports Med. 2012; 40:1742–1749. PMID: 22802273.
Article19. Goedecke A, Wobus M, Krech M, Münch N, Richter K, Hölig K, et al. Differential effect of platelet-rich plasma and fetal calf serum on bone marrow-derived human mesenchymal stromal cells expanded in vitro. J Tissue Eng Regen Med. 2011; 5:648–654. PMID: 21774088.
Article20. Kurita M, Aiba-Kojima E, Shigeura T, Matsumoto D, Suga H, Inoue K, et al. Differential effects of three preparations of human serum on expansion of various types of human cells. Plast Reconstr Surg. 2008; 122:438–448. PMID: 18626359.
Article21. Roussy Y, Bertrand Duchesne MP, Gagnon G. Activation of human platelet-rich plasmas: effect on growth factors release, cell division and in vivo bone formation. Clin Oral Implants Res. 2007; 18:639–648. PMID: 17590158.22. Dohan Ehrenfest DM, Bielecki T, Jimbo R, Barbé G, Del Corso M, Inchingolo F, et al. Do the fibrin architecture and leukocyte content influence the growth factor release of platelet concentrates? An evidence-based answer comparing a pure platelet-rich plasma (P-PRP) gel and a leukocyte-and platelet-rich fibrin (L-PRF). Curr Pharm Biotechnol. 2012; 13:1145–1152. PMID: 21740377.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Characterization of the cytokine profile of platelet rich plasma (PRP) and PRP-induced cell proliferation and migration: Upregulation of matrix metalloproteinase-1 and -9 in HaCaT cells
- Platelet-Rich Fibrin Lysate Can Ameliorate Dysfunction of Chronically UVA-Irradiated Human Dermal Fibroblasts
- The Effects of Immunosuppressants Rapamycin and Cyclosporin A on the Proliferation, Cell Cycle, and Cyclin Expression of Cultured Human Keratinocyte Cell Line HaCaT Cells
- The Effects of Human Bone Marrow-Derived Mesenchymal Stem Cell Conditioned Media Produced with Fetal Bovine Serum or Human Platelet Lysate on Skin Rejuvenation Characteristics
- Human platelet lysate efficiency, stability, and optimal heparin concentration required in culture of mammalian cells