Yonsei Med J.
2010 Jan;51(1):52-57. 10.3349/ymj.2010.51.1.52.
Delivery of Factor VIII Gene into Skeletal Muscle Cells Using Lentiviral Vector
- Affiliations
-
- 1Department of Internal Medicine, Chungbuk National University School of Medicine, Cheongju, Korea. stkim@chungbuk.ac.kr
- KMID: 1779606
- DOI: http://doi.org/10.3349/ymj.2010.51.1.52
Abstract
- PURPOSE
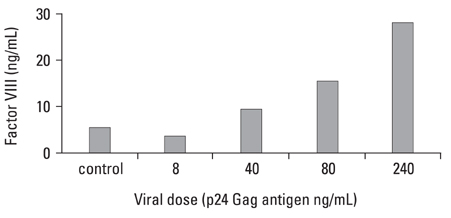
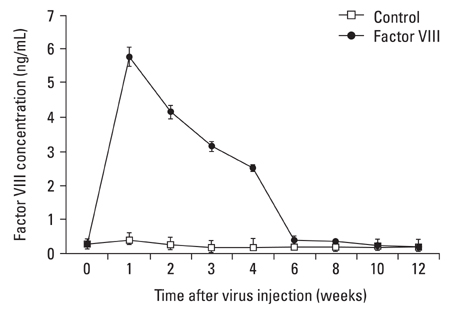
This study was designed to investigate whether transduction of lentiviral vectors (LV) carrying human coagulation factor VIII (hFVIII) cDNA into skeletal muscle could increase circulating hFVIII concentrations. MATERIALS AND METHODS: A LV containing bacterial LacZ gene as a control or human FVIII gene was intramuscularly administered into the thigh muscle of 5 weeks old Sparague-Dawley rats. The plasma human FVIII concentration and neutralizing anti-FVIII antibodies were measured for up to 12 weeks in these experimental animals. RESULTS: The plasma human FVIII levels in the rats injected with LV carrying FVIII cDNA peaked at post-injection 1st week (5.19 +/- 0.14 ng/mL vs. 0.21 +/- 0.05 ng/mL in control rats , p < 0.05). Elevated hFVIII concentrations were maintained for 4 weeks (2.52 +/- 0.83 ng/mL vs. 0.17 +/- 0.08 ng/mL in control rats, p < 0.05) after a single intramuscular injection. In the Bethesda assay, neutralizing antibodies for FVIII protein were detected only in FVIII-LV injected rats by the 10th week, but not in control rats. CONCLUSION: This study suggested that a single administration of an advanced generation LV carrying the human FVIII cDNA resulted in elevation of FVIII level in immune competent rats, and that this gene transfer approach to the skeletal muscle could be an effective tool in treatment of hemophilia A.
MeSH Terms
Figure
Reference
-
1. Kaufman RJ. Advances toward gene therapy for hemophilia at the millennium. Hum Gene Ther. 1999. 10:2091–2107.
Article2. Levine P. Colman R, Hirsh J, Marder V, Saizman E, editors. Clinical manifestations an therapy of hemophilia A and B. Hemostasis and Thrombosis. 1987. 2nd ed. Philadelphia, PA: JB Lippincott;97–111.3. Furie B, Limentani SA, Rosenfield CG. A practical guide to the evaluation and treatment of hemophilia. Blood. 1994. 84:3–9.4. Saenko EL, Ananyeva NM, Shima N, Hauser CA, Pipe SW. The future of recombinant coagulation factors. J Thromb Haemost. 2003. 1:922–930.
Article5. Kreuz W, Ettingshausen CE, Zyschka A, Oldenburg J, Saguer IM, Ehrenforth S, et al. Inhibitor development in previously untreated patients with hemophilia A: a prospective long-term follow-up comparing plasma-derived and recombinant products. Semin Thromb Hemost. 2002. 28:285–290.6. Wion KL, Kelly D, Summerfield JA, Tuddenham EG, Lawn RM. Distribution of factor VIII mRNA and antigen in human liver and other tissues. Nature. 1985. 317:726–729.7. Hollestelle MJ, Thinnes T, Crain K, Stiko A, Kruijt JK, van Berkel TJ, et al. Tissue distribution of factor VIII gene expression in vivo--a closer look. Thromb Haemost. 2001. 86:855–861.8. Zelechowska MG, van Mourik JA, Brodniewicz-Proba T. Ultrastructural localization of factor VIII procoagulant antigen in human liver hepatocytes. Nature. 1985. 317:729–730.
Article9. VandenDriessche T, Collen D, Chuah MK. Viral vector-mediated gene therapy for hemophilia. Curr Gene Ther. 2001. 1:301–315.
Article10. Zufferey R, Nagy D, Mandel RJ, Naldini L, Trono D. Multiply attenuated lentiviral vector achieves efficient gene delivery in vivo. Nat Biotechnol. 1997. 15:871–875.11. Park F, Ohashi K, Chiu W, Naldini L, Kay MA. Efficient lentiviral transduction of liver requires cell cycling in vivo. Nat Genet. 2000. 24:49–52.12. Park F, Ohashi K, Kay MA. Therapeutic levels of human factor VIII and IX using HIV-1-based lentiviral vectors in mouse liver. Blood. 2000. 96:1173–1176.13. Do H, Healey JF, Waller EK, Lollar P. Expression of factor VIII by murine liver sinusoidal endothelial cells. J Biol Chem. 1999. 274:19587–19592.14. Dull T, Zufferey R, Kelly M, Mandel RJ, Nguyen M, Trono D, et al. A third generation lentivirus vector with a conditional packaging system. J Virol. 1998. 72:8463–8471.
Article15. Oh TK, Quan GH, Kim HY, Park F, Kim ST. Correction of anemia in uremic rats by intramuscular injection of lentivirus carrying an erythropoietin gene. Am J Nephrol. 2006. 26:326–334.16. Pittman DD, Alderman EM, Tomkinson KN, Wang JH, Giles AR, Kaufman RJ. Biochemical, immunological, and in vivo functional characterization of B-domain-deleted factor VIII. Blood. 1993. 81:2925–2935.
Article17. Bihoreau N, Paolantonacci P, Bardelle C, Fontaine-Aupart MP, Krishnan S, Yon J, et al. Structural and functional characterization of Factor VIII-delta II, a new recombinant Factor VIII lacking most of the B-domain. Biochem J. 1991. 277:23–31.18. Toole JJ, Pittman DD, Orr EC, Murtha P, Wasley LC, Kaufman RJ. A large region (approximately equal to 95 kDa) of human factor VIII is dispensable for in vitro procoagulant activity. Proc Natl Acad Sci U S A. 1986. 83:5939–5942.19. Ogata K, Mimuro J, Kikuchi J, Tabata T, Ueda Y, Naito M, et al. Expression of human coagulation factor VIII in adipocytes transduced with the simian immunodeficiency virus agm TYO1-based vector for hemophilia A gene therapy. Gene Ther. 2004. 11:253–259.
Article20. Ishiwata A, Mimuro J, Kashiwakura Y, Niimura M, Takano K, Ohmori T, et al. Phenotype correction of hemophilia A mice with adeno-associated virus vectors carrying the B domain-deleted canine factor VIII gene. Thromb Res. 2006. 118:627–635.
Article21. Dull T, Zufferey R, Kelly M, Mandel RJ, Nguyen M, Trono D, et al. A third-generation lentivirus vector with a conditional packaging system. J Virol. 1998. 72:8463–8471.22. Moayeri M, Hawley TS, Hawley RG. Correction of murine hemophilia A by hematopoietic stem cell gene therapy. Mol Ther. 2005. 12:1034–1042.
Article23. Kang Y, Xie L, Tran DT, Stein CS, Hickey M, Davidson BL, et al. Persistent expression of factor VIII in vivo following nonprimate lentiviral gene transfer. Blood. 2005. 106:1552–1558.24. Shi Q, Wilcox DA, Fahs SA, Weiler H, Wells CW, Cooley BC, et al. Factor VIII ectopically targeted to platelets is therapeutic in hemophilia A with high-titer inhibitory antibodies. J Clin Invest. 2006. 116:1974–1982.
Article25. Liu L, Mah C, Fletcher BS. Sustained FVIII expression and phenotypic correction of hemophilia A in neonatal mice using an endothelial-targeted sleeping beauty transposon. Mol Ther. 2006. 13:1006–1015.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- In vivo Erythropoietin Delivery Using Lentiviral Vector in Rats
- In Vitro Study of Adipose-Derived Mesenchymal Stem Cells Transduced with Lentiviral Vector Carrying the Brain-Derived Neurotrophic Factor Gene
- The Validation of RT-PCR Assay for Detection of Replication-competent Lentivirus (RCL) in Vector Preparations Using HIV Vector Based Gene Delivery System
- Dual Expression of Two Transgenes Introduced by Lentiviral Vectors
- Gene Therapy in Rats with a Lentiviral Vector Containing the Human Coagulation Factor IX Gene