Int J Stem Cells.
2020 Nov;13(3):386-393. 10.15283/ijsc20038.
In Vitro Study of Adipose-Derived Mesenchymal Stem Cells Transduced with Lentiviral Vector Carrying the Brain-Derived Neurotrophic Factor Gene
- Affiliations
-
- 1State Key Laboratory of Fine Chemicals, School of Chemical Engineering, Panjin Campus, Dalian University of Technology, Panjin, China
- 2R&D Center of Membrane Science and Technology, School of Chemical Engineering, Dalian University of Technology, Dalian, China
- KMID: 2508912
- DOI: http://doi.org/10.15283/ijsc20038
Abstract
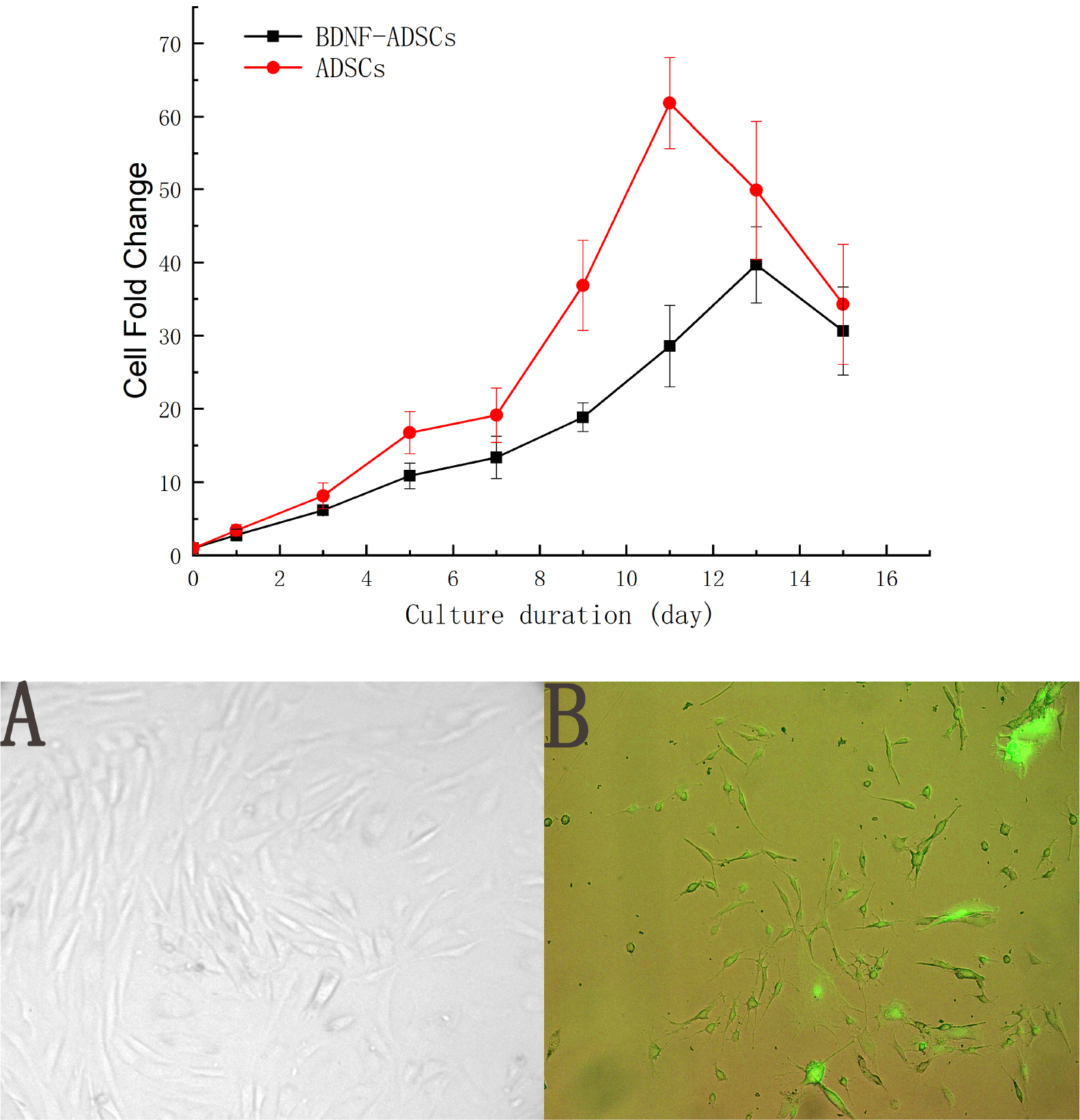
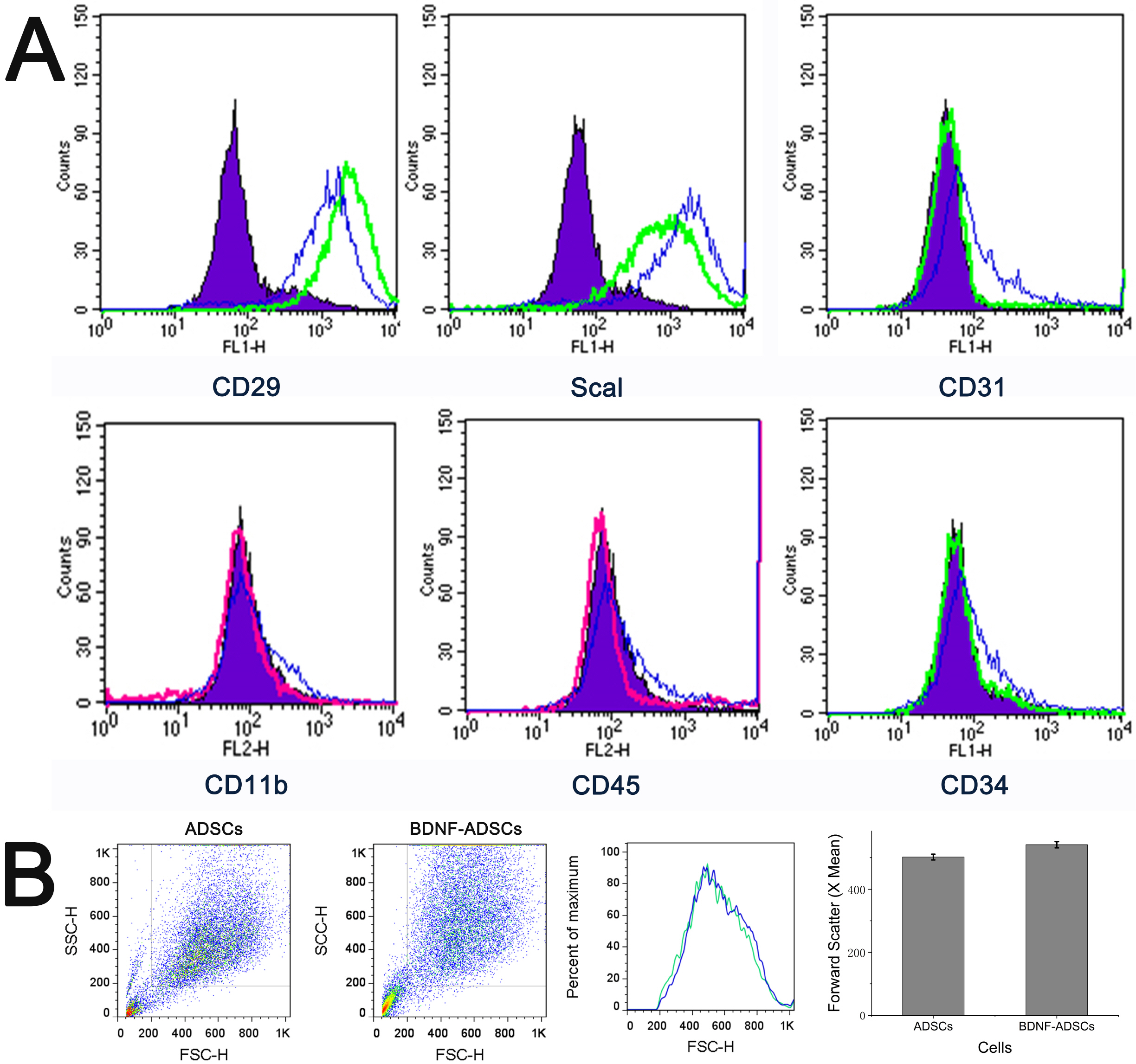
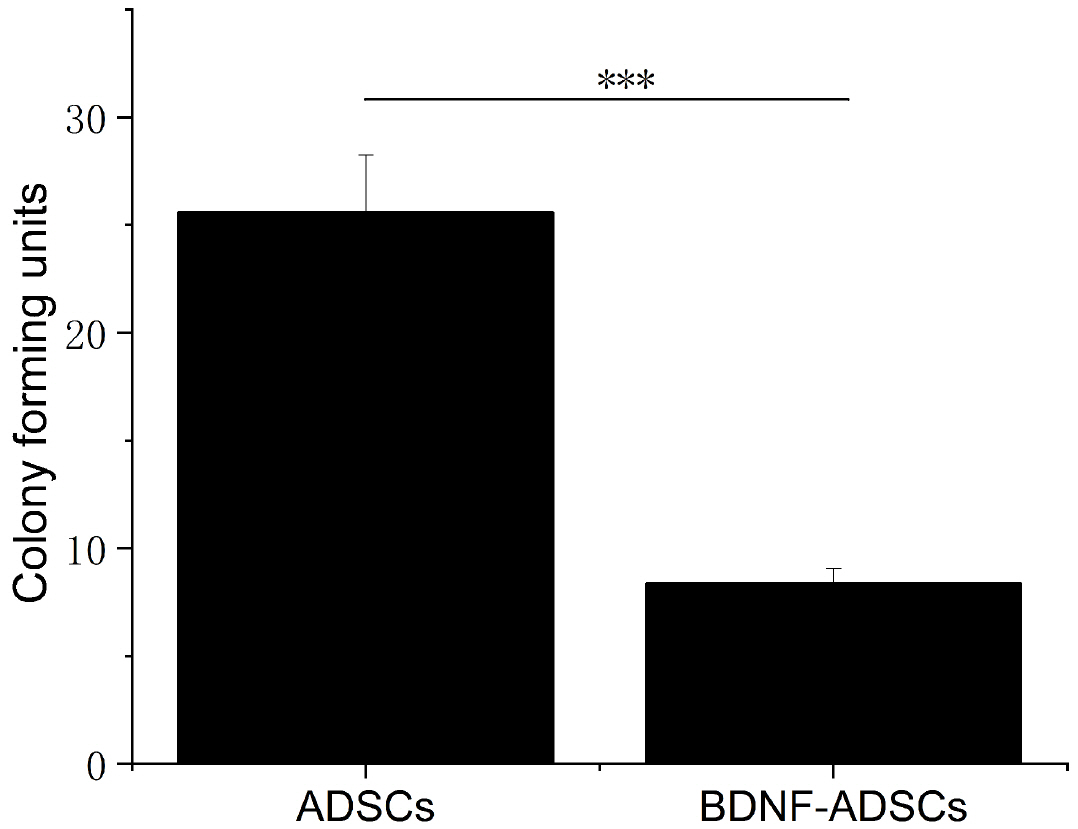
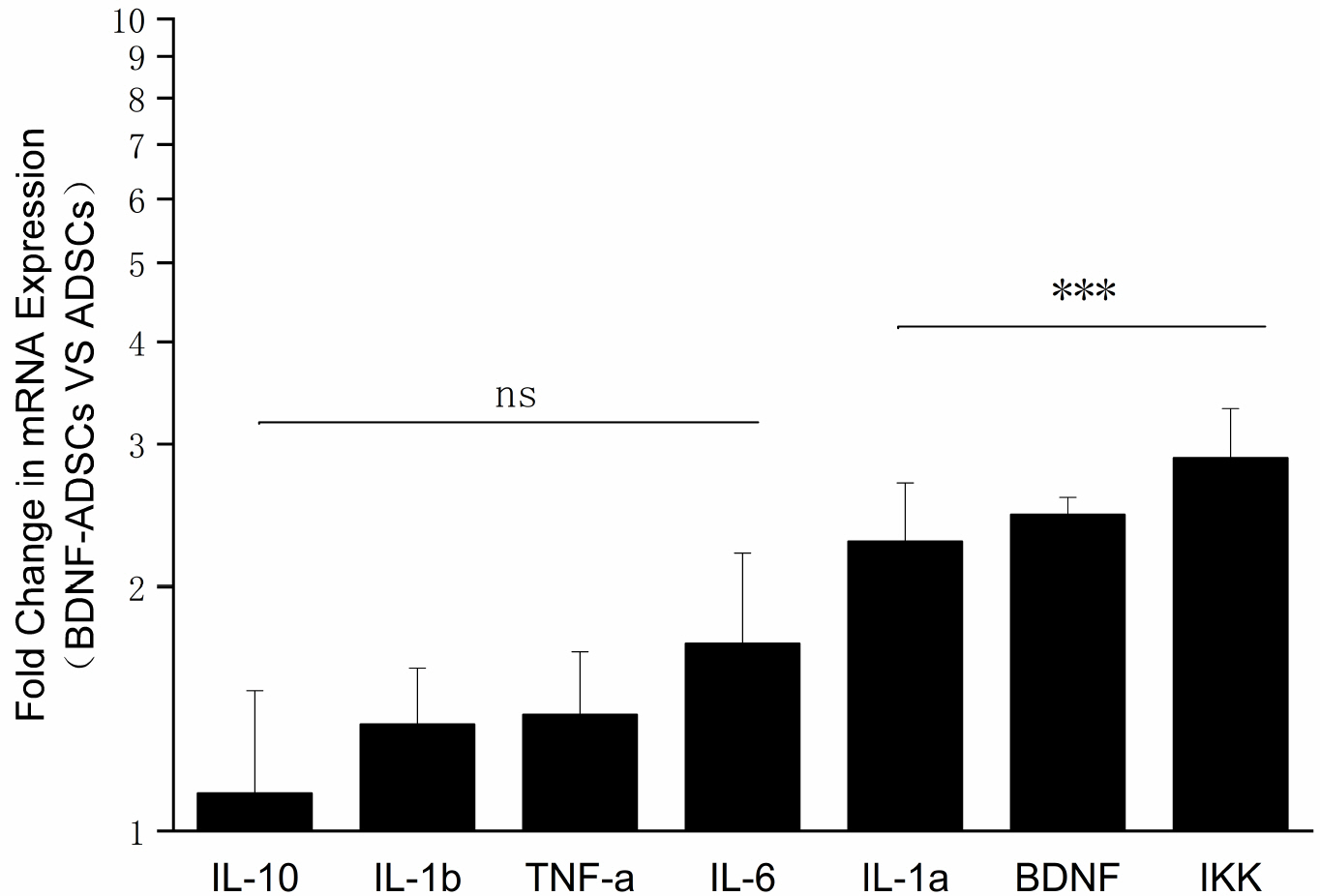
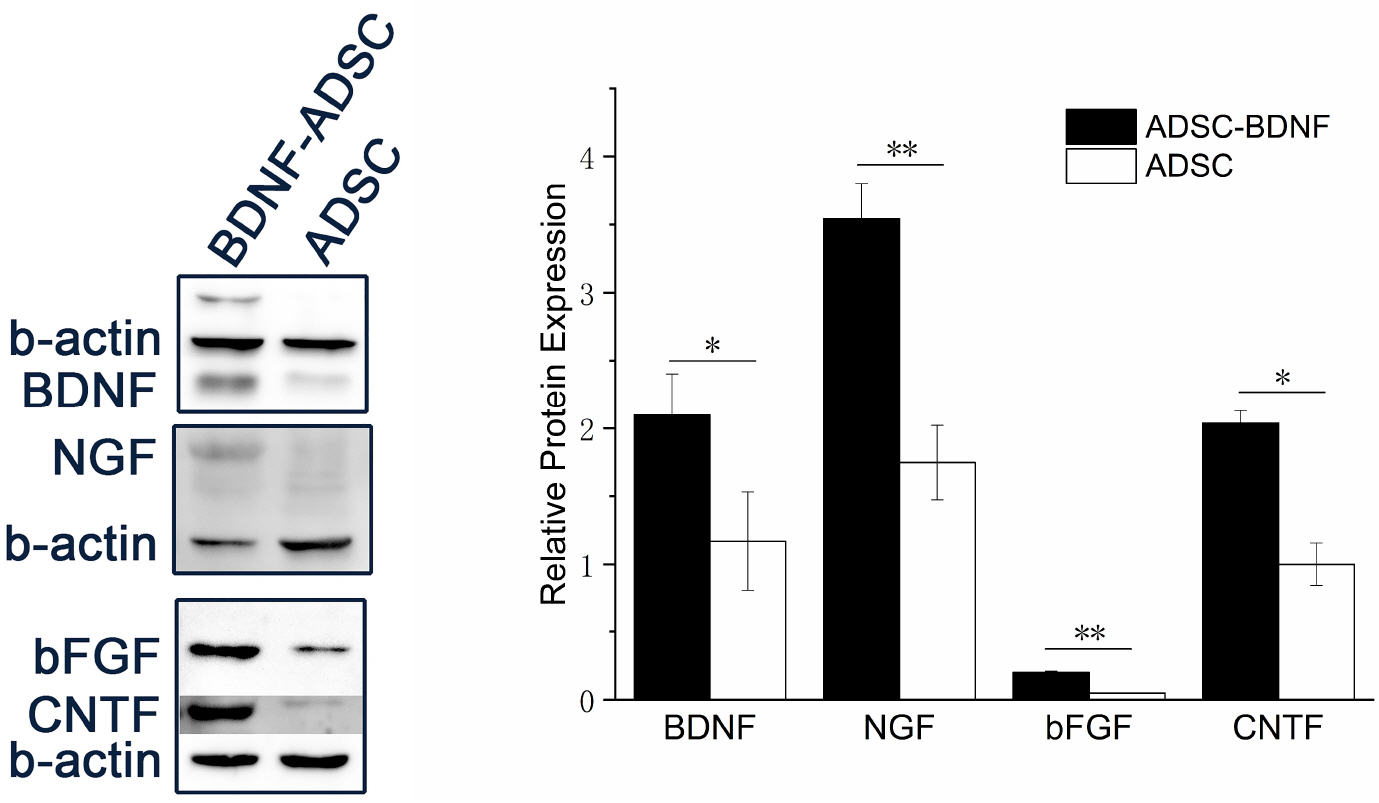
- Brain-derived neurotrophic factor (BDNF) exerts its survival-promoting effects on photoreceptors and retinal ganglion cells, however, delivery systems with little-to-no side effect are needed to sustain its controlled release and long-term efficacy. Our previous studies demonstrated that adipose-derived stem cells (ADSCs) are ideal delivery systems for gene therapy; moreover, ADSCs present unique properties like migration to damaged tissue sites, immunomodulation and anti-inflammation. Herein, we propose to employ ADSCs as the BDNF gene delivery vehicle. Different Analyses like flow cytometry, differentiation and cell proliferation assays etc demonstrated that BDNF were successfully transduced into ADSCs and the stemness of ADSCs was maintained even with the transduction. Real Time PCR and Western blot were used to measure mRNA and protein expressions of the BDNF-transduced ADSCs. The results demonstrated that the BDNF expression level of the lentiviral-BDNF transduced ADSCs is significantly increased and, moreover, enhanced the expression of other neurotrophic and downstream signaling factors. The data suggest that ADSCs are a reliable delivery vehicle for BDNF and could be used for the treatment of various diseases.
Keyword
Figure
Reference
-
References
1. Chen R, Yin XB, Peng CX, Li GL. 2012; Effect of brain-derived neurotrophic factor on c-jun expression in the rd mouse retina. Int J Ophthalmol. 5:266–271. DOI: 10.3980/j.issn.2222-3959.2012.03.03. PMID: 22773970. PMCID: PMC3388390.2. Ernfors P, Wetmore C, Olson L, Persson H. 1990; Identification of cells in rat brain and peripheral tissues expressing mRNA for members of the nerve growth factor family. Neuron. 5:511–526. DOI: 10.1016/0896-6273(90)90090-3. PMID: 2206535.
Article3. Nibuya M, Morinobu S, Duman RS. 1995; Regulation of BDNF and trkB mRNA in rat brain by chronic electroconvulsive seizure and antidepressant drug treatments. J Neurosci. 15:7539–7547. DOI: 10.1523/JNEUROSCI.15-11-07539.1995. PMID: 7472505. PMCID: PMC6578063.
Article4. Amendola T, Fiore M, Aloe L. 2003; Postnatal changes in nerve growth factor and brain derived neurotrophic factor levels in the retina, visual cortex, and geniculate nucleus in rats with retinitis pigmentosa. Neurosci Lett. 345:37–40. DOI: 10.1016/S0304-3940(03)00491-9. PMID: 12809983.
Article5. Mannermaa E, Vellonen KS, Urtti A. 2006; Drug transport in corneal epithelium and blood-retina barrier: emerging role of transporters in ocular pharmacokinetics. Adv Drug Deliv Rev. 58:1136–1163. DOI: 10.1016/j.addr.2006.07.024. PMID: 17081648.
Article6. Tao W, Wen R, Goddard MB, Sherman SD, O'Rourke PJ, Stabila PF, Bell WJ, Dean BJ, Kauper KA, Budz VA, Tsiaras WG, Acland GM, Pearce-Kelling S, Laties AM, Aguirre GD. 2002; Encapsulated cell-based delivery of CNTF reduces photoreceptor degeneration in animal models of retinitis pigmentosa. Invest Ophthalmol Vis Sci. 43:3292–3298. PMID: 12356837.7. Tian C, Weng CC, Yin ZQ. 2010; BDNF improves the efficacy ERG amplitude maintenance by transplantation of retinal stem cells in RCS rats. Adv Exp Med Biol. 664:375–384. DOI: 10.1007/978-1-4419-1399-9_43. PMID: 20238038.
Article8. Dominici M, Le Blanc K, Mueller I, Slaper-Cortenbach I, Marini F, Krause D, Deans R, Keating A, Prockop Dj, Horwitz E. 2006; Minimal criteria for defining multipotent mesenchymal stromal cells. The International Society for Cellular Therapy position statement. Cytotherapy. 8:315–317. DOI: 10.1080/14653240600855905. PMID: 16923606.
Article9. Semon JA, Zhang X, Pandey AC, Alandete SM, Maness C, Zhang S, Scruggs BA, Strong AL, Sharkey SA, Beuttler MM, Gimble JM, Bunnell BA. 2013; Administration of murine stromal vascular fraction ameliorates chronic experimental autoimmune encephalomyelitis. Stem Cells Transl Med. 2:789–796. DOI: 10.5966/sctm.2013-0032. PMID: 23981726. PMCID: PMC3785263.
Article10. Jin HJ, Bae YK, Kim M, Kwon SJ, Jeon HB, Choi SJ, Kim SW, Yang YS, Oh W, Chang JW. 2013; Comparative analysis of human mesenchymal stem cells from bone marrow, adipose tissue, and umbilical cord blood as sources of cell therapy. Int J Mol Sci. 14:17986–18001. DOI: 10.3390/ijms140917986. PMID: 24005862. PMCID: PMC3794764.
Article11. Jankowiak W, Kruszewski K, Flachsbarth K, Skevas C, Richard G, Rüther K, Braulke T, Bartsch U. 2015; Sustained neural stem cell-based intraocular delivery of CNTF attenuates photoreceptor loss in the nclf mouse model of neuronal ceroid lipofuscinosis. PLoS One. 10:e0127204. DOI: 10.1371/journal.pone.0127204. PMID: 25992714. PMCID: PMC4439090.
Article12. Kishimoto S, Inoue K, Nakamura S, Hattori H, Ishihara M, Sakuma M, Toyoda S, Iwaguro H, Taguchi I, Inoue T, Yoshida K. 2016; Low-molecular weight heparin protamine complex augmented the potential of adipose-derived stromal cells to ameliorate limb ischemia. Atherosclerosis. 249:132–139. DOI: 10.1016/j.atherosclerosis.2016.04.003. PMID: 27100923.
Article13. Utsunomiya T, Shimada M, Imura S, Morine Y, Ikemoto T, Mori H, Hanaoka J, Iwahashi S, Saito Y, Iwaguro H. 2011; Human adipose-derived stem cells: potential clinical applications in surgery. Surg Today. 41:18–23. DOI: 10.1007/s00595-010-4415-9. PMID: 21191687.
Article14. Mehrabani D, Mehrabani G, Zare S, Manafi A. 2013; Adipose-derived stem cells (ADSC) and aesthetic surgery: a mini review. World J Plast Surg. 2:65–70. PMID: 25489507. PMCID: PMC4238346.15. Braun J, Kurtz A, Barutcu N, Bodo J, Thiel A, Dong J. 2013; Concerted regulation of CD34 and CD105 accompanies mesenchymal stromal cell derivation from human adventitial stromal cell. Stem Cells Dev. 22:815–827. DOI: 10.1089/scd.2012.0263. PMID: 23072708.
Article16. Zhang X, Bowles AC, Semon JA, Scruggs BA, Zhang S, Strong AL, Gimble JM, Bunnell BA. 2014; Transplantation of autologous adipose stem cells lacks therapeutic efficacy in the experimental autoimmune encephalomyelitis model. PLoS One. 9:e85007. DOI: 10.1371/journal.pone.0085007. PMID: 24465465. PMCID: PMC3897387.
Article17. Connor RI, Mohri H, Cao Y, Ho DD. 1993; Increased viral burden and cytopathicity correlate temporally with CD4+ T-lymphocyte decline and clinical progression in human immunodeficiency virus type 1-infected individuals. J Virol. 67:1772–1777. DOI: 10.1128/JVI.67.4.1772-1777.1993. PMID: 8095306. PMCID: PMC240220.
Article18. Mastrangeli A, Danel C, Rosenfeld MA, Stratford-Perricaudet L, Perricaudet M, Pavirani A, Lecocq JP, Crystal RG. 1993; Diversity of airway epithelial cell targets for in vivo recombinant adenovirus-mediated gene transfer. J Clin Invest. 91:225–234. DOI: 10.1172/JCI116175. PMID: 8423221. PMCID: PMC330018.
Article19. Madonna R, Bolli R, Rokosh G, De Caterina R. 2013; Long-term engraftment and angiogenic properties of lentivirally transduced adipose tissue-derived stromal cells. Mol Biotechnol. 54:13–24. DOI: 10.1007/s12033-012-9537-4. PMID: 22492300.
Article20. Rider P, Carmi Y, Voronov E, Apte RN. 2013; Interleukin-1α. Semin Immunol. 25:430–438. DOI: 10.1016/j.smim.2013.10.005. PMID: 24183701.21. Dinarello CA. 2011; Interleukin-1 in the pathogenesis and treatment of inflammatory diseases. Blood. 117:3720–3732. DOI: 10.1182/blood-2010-07-273417. PMID: 21304099. PMCID: PMC3083294.
Article22. Bertheloot D, Latz E. 2017; HMGB1, IL-1α, IL-33 and S100 proteins: dual-function alarmins. Cell Mol Immunol. 14:43–64. DOI: 10.1038/cmi.2016.34. PMID: 27569562. PMCID: PMC5214941.
Article23. Redondo-Castro E, Cunningham C, Miller J, Martuscelli L, Aoulad-Ali S, Rothwell NJ, Kielty CM, Allan SM, Pinteaux E. 2017; Interleukin-1 primes human mesenchymal stem cells towards an anti-inflammatory and pro-trophic phenotype in vitro. Stem Cell Res Ther. 8:79. DOI: 10.1186/s13287-017-0531-4. PMID: 28412968. PMCID: PMC5393041.
Article24. Barbacid M. 1994; The Trk family of neurotrophin receptors. J Neurobiol. 25:1386–1403. DOI: 10.1002/neu.480251107. PMID: 7852993.
Article25. Hetman M, Kanning K, Cavanaugh JE, Xia Z. 1999; Neuropro-tection by brain-derived neurotrophic factor is mediated by extracellular signal-regulated kinase and phosphatidylinositol 3-kinase. J Biol Chem. 274:22569–22580. DOI: 10.1074/jbc.274.32.22569. PMID: 10428835.
Article26. Wilson RB, Kunchithapautham K, Rohrer B. 2007; Paradoxical role of BDNF: BDNF+/-retinas are protected against light damage-mediated stress. Invest Ophthalmol Vis Sci. 48:2877–2886. DOI: 10.1167/iovs.06-1079. PMID: 17525224. PMCID: PMC1964504.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Neural Differentiation of Bone Marrow-Derived Mesenchymal Stem Cells: Applicability for Inner Ear Therapy
- Concise Review: Differentiation of Human Adult Stem Cells Into Hepatocyte-like Cells In vitro
- The Effect of Recombinant Tyrosine Hydroxylase Expression on the Neurogenic Differentiation Potency of Mesenchymal Stem Cells
- Increased Expression of Brain-derived Neurotrophic Factor in Irritable Bowel Syndrome and Its Correlation With Abdominal Pain (Gut 2012;61:685-694)
- Therapeutic Angiogenesis with Somatic Stem Cell Transplantation