Korean J Pain.
2013 Jan;26(1):32-38. 10.3344/kjp.2013.26.1.32.
Continuous Intrathecal Morphine Administration for Cancer Pain Management Using an Intrathecal Catheter Connected to a Subcutaneous Injection Port: A Retrospective Analysis of 22 Terminal Cancer Patients in Korean Population
- Affiliations
-
- 1Department of Anesthesiology and Pain Medicine, School of Medicine, Catholic University of Daegu, Daegu, Korea. usmed@cu.ac.kr
- KMID: 1779021
- DOI: http://doi.org/10.3344/kjp.2013.26.1.32
Abstract
- BACKGROUND
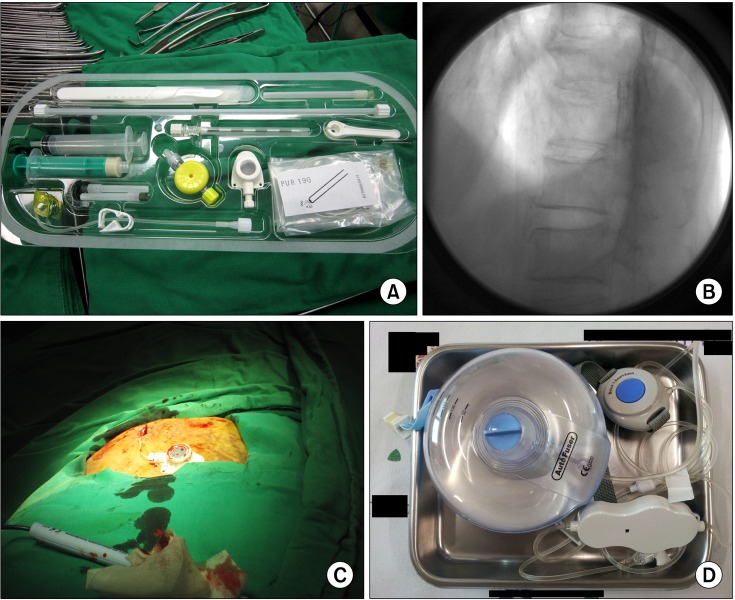
Intrathecal opioid administration has been used widely in patients suffering from severe cancer pain that is not managed with conventional modalities. However, the potential serious neurological complications from the procedure and the side effects of intrathecal opioids have made many clinicians reluctant to employ continuous intrathecal analgesia as a first-line therapeutic option despite its dramatic effect on intractable pain. We retrospectively investigated the efficacy, side effects, and complications of intrathecal morphine administration through intrathecal catheters connected to a subcutaneous injection port (ICSP) in 22 Korean terminal cancer patients with successful intrathecal morphine trials.
METHODS
Patient demographic data, the duration of intrathecal opioid administration, preoperative numerical pain rating scales (NRS) and doses of systemic opioids, side effects and complications related to intrathecal opioids and the procedure, and the numerical pain rating scales and doses of intrathecal and systemic opioids on the 1st, 3rd, 7th and 30th postoperative days were determined from medical records.
RESULTS
Intrathecal morphine administration for 46.0 +/- 61.3 days significantly reduced NRS from baseline on all the postoperative days. A significant increase in intrathecal opioids with a nonsignificant decrease in systemic opioids was observed on the 7th and 30th postoperative days compared to the 1st postoperative day. The most common side effects of intrathecal opioids were nausea/vomiting (31.8%) and urinary retention (38.9%), which were managed with conservative therapies.
CONCLUSIONS
Intrathecal morphine administration using ICSP provided immediate and beneficial effects on pain scores with tolerable side effects in terminal cancer patients.
Keyword
MeSH Terms
Figure
Cited by 2 articles
-
Role of Catheter's Position for Final Results in Intrathecal Drug Delivery. Analysis Based on CSF Dynamics and Specific Drugs Profiles
De Andres Jose, Perotti Luciano, Villanueva Vicente, Asensio Samper Juan Marcos, Fabregat-Cid Gustavo
Korean J Pain. 2013;26(4):336-346. doi: 10.3344/kjp.2013.26.4.336.Epidural Infusion of Morphine and Levobupivacaine through a Subcutaneous Port for Cancer Pain Management
Bong Ha Heo, Tae Hee Pyeon, Hyung Gon Lee, Woong Mo Kim, Jeong Il Choi, Myung Ha Yoon
Korean J Pain. 2014;27(2):139-144. doi: 10.3344/kjp.2014.27.2.139.
Reference
-
1. Holmfred A, Vikerfors T, Berggren L, Gupta A. Intrathecal catheters with subcutaneous port systems in patients with severe cancer-related pain managed out of hospital: the risk of infection. J Pain Symptom Manage. 2006; 31:568–572. PMID: 16793497.
Article2. Vissers KC, Besse K, Wagemans M, Zuurmond W, Giezeman MJ, Lataster A, et al. 23. Pain in patients with cancer. Pain Pract. 2011; 11:453–475. PMID: 21679293.3. Smith TJ, Staats PS, Deer T, Stearns LJ, Rauck RL, Boortz-Marx RL, et al. Randomized clinical trial of an implantable drug delivery system compared with comprehensive medical management for refractory cancer pain: impact on pain, drug-related toxicity, and survival. J Clin Oncol. 2002; 20:4040–4049. PMID: 12351602.
Article4. Deer TR, Smith HS, Burton AW, Pope JE, Doleys DM, Levy RM, et al. Comprehensive consensus based guidelines on intrathecal drug delivery systems in the treatment of pain caused by cancer pain. Pain Physician. 2011; 14:E283–E312. PMID: 21587338.5. Hong SH, Lee GW. Upper body cancer pain management by cervical intrathecal catheterization: a case report. Korean J Anesthesiol. 2008; 55:135–138.
Article6. Seo KC, Chung JY, Kim HY, Rho WS, Kim BI, Song SY. Intrathecal catheter and subcutaneous access port implantation in pain management for terminal cancer patient: a case report. Korean J Pain. 2007; 20:240–245.
Article7. Mercadante S, Caraceni A. Conversion ratios for opioid switching in the treatment of cancer pain: a systematic review. Palliat Med. 2011; 25:504–515. PMID: 21708857.
Article8. Rauck RL, Cherry D, Boyer MF, Kosek P, Dunn J, Alo K. Long-term intrathecal opioid therapy with a patient-activated, implanted delivery system for the treatment of refractory cancer pain. J Pain. 2003; 4:441–447. PMID: 14622664.
Article9. Becker R, Jakob D, Uhle EI, Riegel T, Bertalanffy H. The significance of intrathecal opioid therapy for the treatment of neuropathic cancer pain conditions. Stereotact Funct Neurosurg. 2000; 75:16–26. PMID: 11416261.
Article10. Angst MS, Clark JD. Opioid-induced hyperalgesia: a qualitative systematic review. Anesthesiology. 2006; 104:570–587. PMID: 16508405.
Article11. Krames ES. Intrathecal infusional therapies for intractable pain: patient management guidelines. J Pain Symptom Manage. 1993; 8:36–46. PMID: 8482892.
Article12. Belverud S, Mogilner A, Schulder M. Intrathecal pumps. Neurotherapeutics. 2008; 5:114–122. PMID: 18164490.
Article13. Aldrete JA. Intrathecal opioid infusions. Anesthesiology. 2004; 101:256. author reply 257-8. PMID: 15220803.
Article14. Anderson VC, Burchiel KJ. A prospective study of long-term intrathecal morphine in the management of chronic nonmalignant pain. Neurosurgery. 1999; 44:289–300. discussion 300-1. PMID: 9932882.
Article15. Bailey PL, Rhondeau S, Schafer PG, Lu JK, Timmins BS, Foster W, et al. Dose-response pharmacology of intrathecal morphine in human volunteers. Anesthesiology. 1993; 79:49–59. discussion 25A. PMID: 8342828.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Intrathecal Catheter and Subcutaneous Access Port Implantation in Pain Management for Terminal Cancer Patient: A case report
- Pain Management of Terminal Cancer Patients by Intrathecal Injection of Local Anesthetics, Opioid and Adjuvants
- Epidural Infusion of Morphine and Levobupivacaine through a Subcutaneous Port for Cancer Pain Management
- Upper body cancer pain management by cervical intrathecal catheterization: A case report
- Continuous Intraventricular Morphine Infusion for Control of Pain in Terminal Cancer Patients