J Korean Med Sci.
2010 Mar;25(3):399-404. 10.3346/jkms.2010.25.3.399.
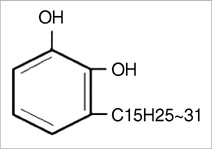
In vitro Antibacterial and Morphological Effects of the Urushiol Component of the Sap of the Korean lacquer tree (Rhus vernicifera Stokes) on Helicobacter pylori
- Affiliations
-
- 1Department of Internal Medicine, Hallym University College of Medicine, Chuncheon, Korea.
- 2Department of Internal Medicine, Institute of Lifelong Health, Wonju Rhus Project Team, Yonsei University Wonju College of Medicine, Wonju, Korea. baiksk@medimail.co.kr
- 3Department of Laboratory Medicine, Institute of Lifelong Health, Wonju Rhus Project Team, Yonsei University Wonju College of Medicine, Wonju, Korea.
- 4Department of Microbiology, Institute of Lifelong Health, Wonju Rhus Project Team, Yonsei University Wonju College of Medicine, Wonju, Korea.
- 5Department of Dermatology, Institute of Lifelong Health, Wonju Rhus Project Team, Yonsei University Wonju College of Medicine, Wonju, Korea.
- 6College of Agricultural and Life Science, Gangwon National University, Chuncheon, Korea.
- 7Department of Microbiology, Gyeongsang National University School of Medicine, Jinju, Korea.
- KMID: 1778035
- DOI: http://doi.org/10.3346/jkms.2010.25.3.399
Abstract
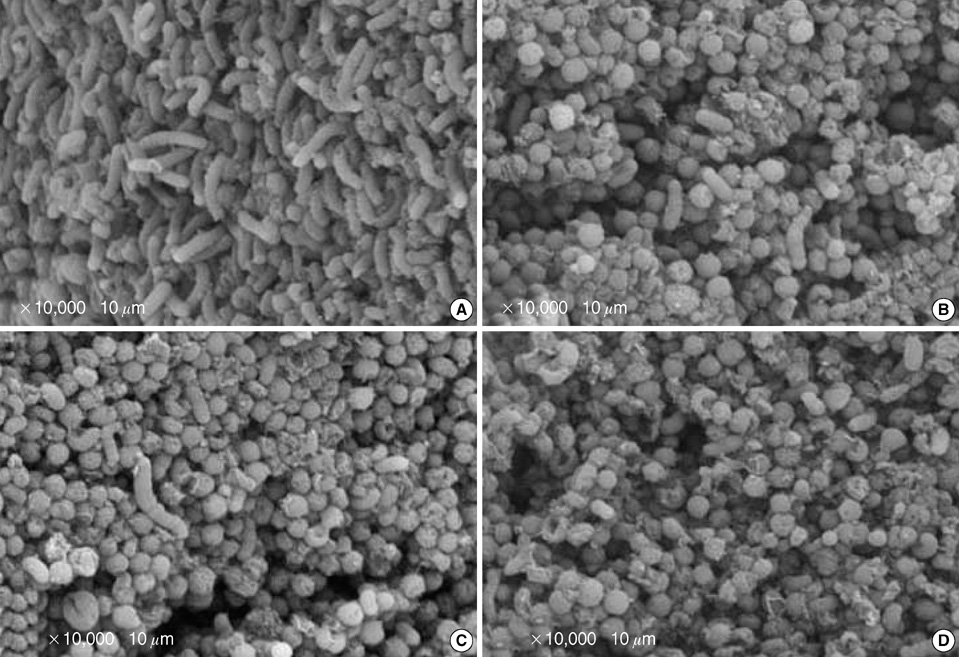
- Eradication regimens for Helicobacter pylori infection have some side effects, compliance problems, relapses, and antibiotic resistance. Therefore, alternative anti-H. pylori or supportive antimicrobial agents with fewer disadvantages are necessary for the treatment of H. pylori. We investigated the pH-(5.0, 6.0, 7.0, 8.0, 9.0, and 10.0) and concentration (0.032, 0.064, 0.128, 0.256, 0.514, and 1.024 mg/mL)-dependent antibacterial activity of crude urushiol extract from the sap of the Korean lacquer tree (Rhus vernicifera Stokes) against 3 strains (NCTC11637, 69, and 219) of H. pylori by the agar dilution method. In addition, the serial (before incubation, 3, 6, and 10 min after incubation) morphological effects of urushiol on H. pylori were examined by electron microscopy. All strains survived only within pH 6.0-9.0. The minimal inhibitory concentrations of the extract against strains ranged from 0.064 mg/mL to 0.256 mg/mL. Urushiol caused mainly separation of the membrane, vacuolization, and lysis of H. pylori. Interestingly, these changes were observed within 10 min following incubation with the 1 x minimal inhibitory concentrations of urushiol. The results of this work suggest that urushiol has potential as a rapid therapeutic against H. pylori infection by disrupting the bacterial cell membrane.
Keyword
MeSH Terms
-
Anti-Bacterial Agents/chemistry/*pharmacology/therapeutic use
Catechols/chemistry/*pharmacology/therapeutic use
Cell Membrane/drug effects/ultrastructure
Helicobacter Infections/drug therapy
Helicobacter pylori/*drug effects/ultrastructure
Humans
Microbial Sensitivity Tests
Microbial Viability/drug effects
Molecular Structure
Rhus/*chemistry
Anti-Bacterial Agents
Catechols
Figure
Cited by 1 articles
-
Antibacterial effect of urushiol on E. faecalis as a root canal irrigant
Sang-Wan Kim, Dong-Hoon Shin
Restor Dent Endod. 2017;42(1):54-59. doi: 10.5395/rde.2017.42.1.54.
Reference
-
1. Ferreira AC, Isomoto H, Moriyama M, Fujioka T, Machado JC, Yamaoka Y. Helicobacter and gastric malignancies. Helicobacter. 2008. 13:Suppl 1. 28–34.2. Kandulski A, Selgrad M, Malfertheiner P. Helicobacter pylori infection: a clinical overview. Dig Liver Dis. 2008. 40:619–626.
Article3. Marzio L, Cellini L, Angelucci D. Triple therapy for 7 days vs. triple therapy for 7 days plus omeprazole for 21 days in treatment of active duodenal ulcer with Helicobacter pylori infection. A double blind placebo controlled trial. Dig Liver Dis. 2003. 35:20–23.4. Malfertheiner P, Megraud F, O'Morain C, Bazzoli F, El-Omar E, Graham D, Hunt R, Rokkas T, Vakil N, Kuipers EJ. Current concepts in the management of Helicobacter pylori infection: the Maastricht III Consensus Report. Gut. 2007. 56:772–781.
Article5. Elsohly MA, Adawadkar PD, Benigni DA, Watson ES, Little TL Jr. Analogues of poison ivy urushiol. Synthesis and biological activity of disubstituted n-alkylbenzenes. J Med Chem. 1986. 29:606–611.6. Kim MJ, Choi YH, Kim WG, Kwak SS. Antioxidative activity of urushiol derivatives from the sap of lacquer tree. Korean J Plant Resour. 1997. 10:227–230.7. Kim MJ, Kim CJ, Kwak SS. Antifungal activity of urushiol component in the sap of Korean lacquer tree (Rhus vernicifera Stokes). Korean J Plant Resour. 1997. 10:231–234.8. Horii T, Mase K, Suzuki Y, Kimura T, Ohta M, Maekawa M, Kanno T, Kobayashi M. Antibacterial activities of beta-lactamase inhibitors associated with morphological changes of cell wall in Helicobacter pylori. Helicobacter. 2002. 7:39–45.
Article9. Adeniyi BA, Anyiam FM. In vitro anti-Helicobacter pylori potential of methanol extract of Allium ascalonicum Linn. (Liliaceae) leaf: susceptibility and effect on urease activity. Phytother Res. 2004. 18:358–361.
Article10. Tecder-Unal M, Can F, Demirbilek M, Karabay G, Tufan H, Arslan H. The bactericidal and morphological effects of peroxynitrite on Helicobacter pylori. Helicobacter. 2008. 13:42–48.11. Stamatis G, Kyriazopoulos P, Golegou S, Basayiannis A, Skaltsas S, Skaltsa H. In vitro anti-Helicobacter pylori activity of Greek herbal medicines. J Ethnopharmacol. 2003. 88:175–179.
Article12. Kamiji MM, de Oliveira RB. Non-antibiotic therapies for Helicobacter pylori infection. Eur J Gastroenterol Hepatol. 2005. 17:973–981.
Article13. Malekzadeh F, Ehsanifar H, Shahamat M, Levin M, Colwell RR. Antibacterial activity of black myrobalan (Terminalia chebula Retz) against Helicobacter pylori. Int J Antimicrob Agents. 2001. 18:85–88.
Article14. Nostro A, Cellini L, Di Bartolomeo S, Di Campli E, Grande R, Cannatelli MA, Marzio L, Alonzo V. Antibacterial effect of plant extracts against Helicobacter pylori. Phytother Res. 2005. 19:198–202.15. Lascols C, Bryskier A, Soussy CJ, Tankovic J. Effect of pH on the susceptibility of Helicobacter pylori to the ketolide telithromycin (HMR 3647) and clarithromycin. J Antimicrob Chemother. 2001. 48:738–740.16. Hassan IJ, Stark RM, Greenman J, Millar MR. Activities of beta-lactams and macrolides against Helicobacter pylori. Antimicrob Agents Chemother. 1999. 43:1387–1392.17. McGowan CC, Cover TL, Blaser MJ. Helicobacter pylori and gastric acid: biological and therapeutic implications. Gastroenterology. 1996. 110:926–938.
Article18. Park MJ, Kim JS, Yim JY, Jung HC, Song IS, Yu ES, Lee JJ, Huh CS, Baek YJ. The suppressive effect of a fermented milk containing lactobacilli on Helicobacter pylori in human gastric mucosa. Korean J Gastroenterol. 2001. 38:233–240.19. Wang YC, Huang TL. Anti-Helicobacter pylori activity of Plumbago zeylanica L. FEMS Immunol Med Microbiol. 2005. 43:407–412.20. Millar MR, Pike J. Bactericidal activity of antimicrobial agents against slowly growing Helicobacter pylori. Antimicrob Agents Chemother. 1992. 36:185–187.
Article21. Blacky A, Makristathis A, Apfalter P, Willinger B, Rotter ML, Hirschl AM. In vitro activity of fosfomycin alone and in combination with amoxicillin, clarithromycin and metronidazole against Helicobacter pylori compared with combined clarithromycin and metronidazole. Eur J Clin Microbiol Infect Dis. 2005. 24:276–279.
Article22. Xia Z, Miyakoshi T, Yoshida T. Lipoxygenase-catalyzed polymerization of phenolic lipids suggests a new mechanism for allergic contact dermatitis induced by urushiol and its analogs. Biochem Biophys Res Commun. 2004. 315:704–709.
Article23. Harigaya S, Honda T, Rong L, Miyakoshi T, Chen CL. Enzymatic dehydrogenative polymerization of urushiols in fresh exudates from the lacquer tree, Rhus vernicifera DC. J Agric Food Chem. 2007. 55:2201–2208.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Experimental Study on Latent Sensitivity to Rhus Trees
- A Case of Erythema Multiforme - like Eruptions due to Contact with Lacquer Tree
- A Case of Connubial Contact Dermatitis due to Rhus
- The Biological Effects of Rhus (Japanese lacquer sap) on Rats
- Urushiol V Suppresses Cell Proliferation and Enhances Antitumor Activity of 5-FU in Human Colon Cancer Cells by Downregulating FoxM1