Korean J Physiol Pharmacol.
2012 Jun;16(3):193-198. 10.4196/kjpp.2012.16.3.193.
Proteomic Analysis of Colonic Mucosal Tissue from Tuberculous and Ulcerative Colitis Patients
- Affiliations
-
- 1Department of Physiology, Kwandong University College of Medicine, Gangneung 210-701, Korea.
- 2Department of Physiology, School of Medicine, Konkuk University, Chungju 380-701, Korea.
- 3Department of Internal Medicine, University of Ulsan College of Medicine, Gangneung Asan Hospital, Gangneung 210-711, Korea. 9292@gnah.co.kr
- KMID: 1768013
- DOI: http://doi.org/10.4196/kjpp.2012.16.3.193
Abstract
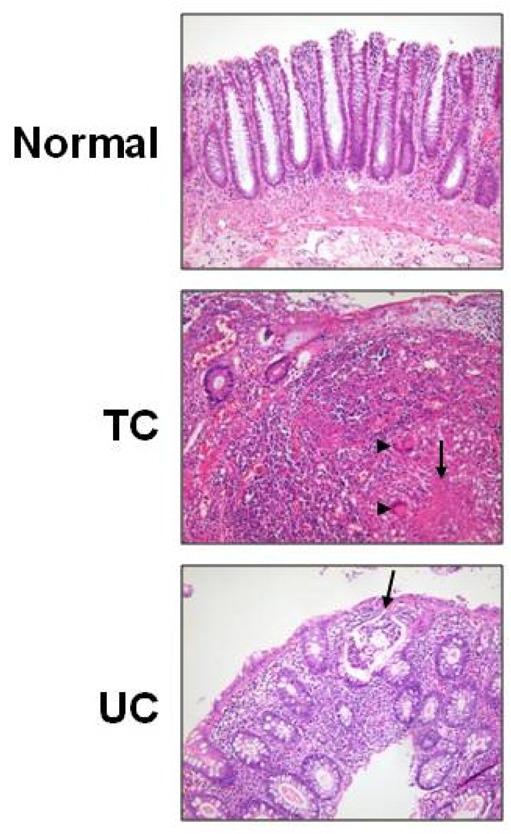
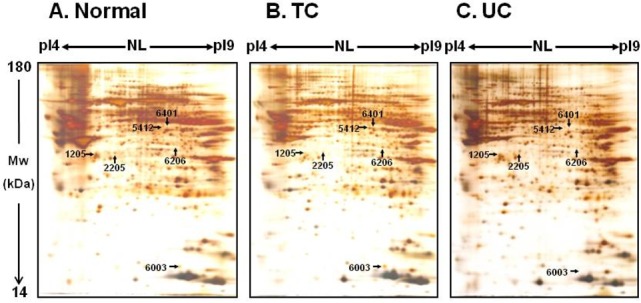
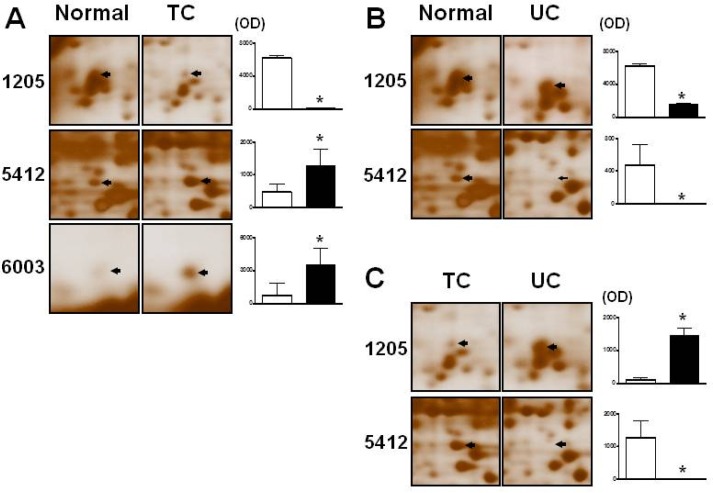
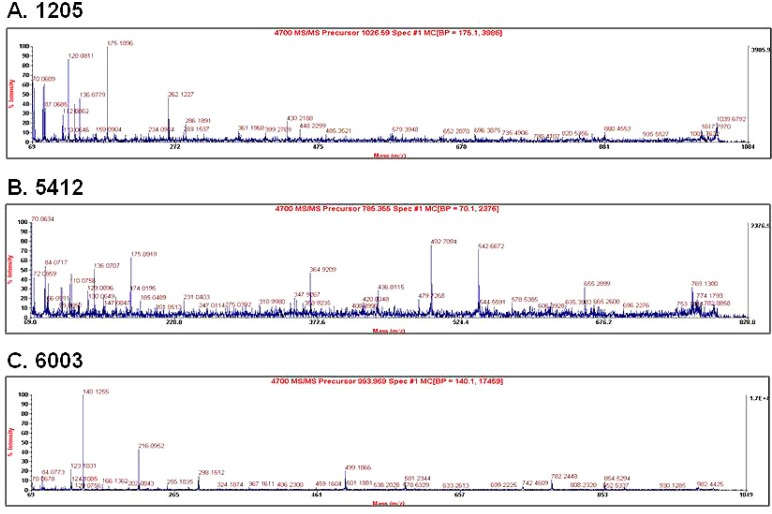
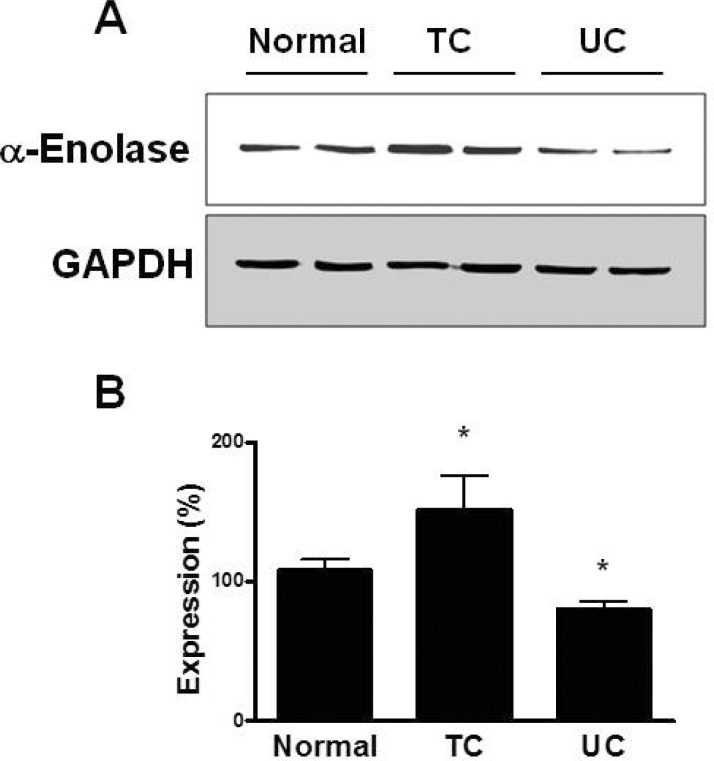
- Changes in the expression profiles of specific proteins leads to serious human diseases, including colitis. The proteomic changes related to colitis and the differential expression between tuberculous (TC) and ulcerative colitis (UC) in colon tissue from colitis patients has not been defined. We therefore performed a proteomic analysis of human TC and UC mucosal tissue. Total protein was obtained from the colon mucosal tissue of normal, TC, and UC patients, and resolved by 2-dimensional electrophoresis (2-DE). The results were analyzed with PDQuest using silver staining. We used matrix-assisted laser desorption ionization time-of-flight/time-of-flight spectrometry (MALDI TOF/TOF) to identify proteins differentially expressed in TC and UC. Of the over 1,000 proteins isolated, three in TC tissue and two in UC tissue displayed altered expression when compared to normal tissue. Moreover, two proteins were differentially expressed in a comparative analysis between TC and UC. These were identified as mutant beta-actin, alpha-enolase and Charcot-Leyden crystal protein. In particular, the expression of alpha-enolase was significantly greater in TC compared with normal tissue, but decreased in comparison to UC, implying that alpha-enolase may represent a biomarker for differential diagnosis of TC and UC. This study therefore provides a valuable resource for the molecular and diagnostic analysis of human colitis.
Keyword
MeSH Terms
-
Actins
Colitis
Colitis, Ulcerative
Colon
Diagnosis, Differential
Electrophoresis
Glycoproteins
Humans
Lysophospholipase
Mucous Membrane
Phosphopyruvate Hydratase
Proteins
Proteomics
Silver Staining
Spectrometry, Mass, Matrix-Assisted Laser Desorption-Ionization
Spectrum Analysis
Ulcer
Actins
Glycoproteins
Lysophospholipase
Phosphopyruvate Hydratase
Proteins
Figure
Reference
-
1. Horn AE, Ufberg JW. Appendicitis, diverticulitis, and colitis. Emerg Med Clin North Am. 2011; 29:347–368. PMID: 21515183.
Article2. Rasheed S, Zinicola R, Watson D, Bajwa A, McDonald PJ. Intra-abdominal and gastrointestinal tuberculosis. Colorectal Dis. 2007; 9:773–783. PMID: 17868413.
Article3. Brenner SM, Annes G, Parker JG. Tuberculous colitis simulating nonspecific granulomatous disease of the colon. Am J Dig Dis. 1970; 15:85–92. PMID: 5410870.
Article4. Danese S, Fiocchi C. Ulcerative colitis. N Engl J Med. 2011; 365:1713–1725. PMID: 22047562.
Article5. Page MJ, Amess B, Rohlff C, Stubberfield C, Parekh R. Proteomics: a major new technology for the drug discovery process. Drug Discov Today. 1999; 4:55–62. PMID: 10234157.
Article6. Mayr U, Mayr M, Yin X, Begum S, Tarelli E, Wait R, Xu Q. Proteomic dataset of mouse aortic smooth muscle cells. Proteomics. 2005; 5:4546–4557. PMID: 16240290.
Article7. Pinet F, Poirier F, Fuchs S, Tharaux PL, Caron M, Corvol P, Michel JB, Joubert-Caron R. Troponin T as a marker of differentiation revealed by proteomic analysis in renal arterioles. FASEB J. 2004; 18:585–586. PMID: 14715693.
Article8. Won KJ, Lee P, Jung SH, Jiang X, Lee CK, Lin HY, Kang H, Lee HM, Kim J, Toyokuni S, Kim B. 3-morpholinosydnonimine participates in the attenuation of neointima formation via inhibition of annexin A2-mediated vascular smooth muscle cell migration. Proteomics. 2011; 11:193–201. PMID: 21204247.
Article9. Lee CK, Han JS, Won KJ, Jung SH, Park HJ, Lee HM, Kim J, Park YS, Kim HJ, Park PJ, Park TK, Kim B. Diminished expression of dihydropteridine reductase is a potent biomarker for hypertensive vessels. Proteomics. 2009; 9:4851–4858. PMID: 19743417.
Article10. Lee CK, Kim HJ, Lee YR, So HH, Park HJ, Won KJ, Park T, Lee KY, Lee HM, Kim B. Analysis of peroxiredoxin decreasing oxidative stress in hypertensive aortic smooth muscle. Biochim Biophys Acta. 2007; 1774:848–855. PMID: 17556052.
Article11. Hsieh SY, Shih TC, Yeh CY, Lin CJ, Chou YY, Lee YS. Comparative proteomic studies on the pathogenesis of human ulcerative colitis. Proteomics. 2006; 6:5322–5331. PMID: 16947118.
Article12. Shkoda A, Werner T, Daniel H, Gunckel M, Rogler G, Haller D. Differential protein expression profile in the intestinal epithelium from patients with inflammatory bowel disease. J Proteome Res. 2007; 6:1114–1125. PMID: 17330946.
Article13. Nagar AB. Isolated colonic ulcers: diagnosis and management. Curr Gastroenterol Rep. 2007; 9:422–428. PMID: 17991345.
Article14. Kühbacher T, Schreiber S, Fölsch UR. Ulcerative colitis: conservative management and long-term effects. Langenbecks Arch Surg. 2004; 389:350–653. PMID: 15133672.
Article15. Minami M, Hasegawa T, Ando T, Maeda O, Ohkura T, Ohta M, Goto H. Post-colonoscopic Listeria septicemia in ulcerative colitis during immunosuppressive therapy. Intern Med. 2007; 46:2023–2027. PMID: 18084128.
Article16. Schroeder KW, Tremaine WJ, Ilstrup DM. Coated oral 5-aminosalicylic acid therapy for mildly to moderately active ulcerative colitis. A randomized study. N Engl J Med. 1987; 317:1625–1629. PMID: 3317057.17. Alvares JF, Devarbhavi H, Makhija P, Rao S, Kottoor R. Clinical, colonoscopic, and histological profile of colonic tuberculosis in a tertiary hospital. Endoscopy. 2005; 37:351–356. PMID: 15824946.
Article19. Vermeulen N, Arijs I, Joossens S, Vermeire S, Clerens S, Van den Bergh K, Michiels G, Arckens L, Schuit F, Van Lommel L, Rutgeerts P, Bossuyt X. Anti-alpha-enolase antibodies in patients with inflammatory Bowel disease. Clin Chem. 2008; 54:534–541. PMID: 18218721.20. Zhao X, Kang B, Lu C, Liu S, Wang H, Yang X, Chen Y, Jiang B, Zhang J, Lu Y, Zhi F. Evaluation of p38 MAPK pathway as a molecular signature in ulcerative colitis. J Proteome Res. 2011; 10:2216–2225. PMID: 21428429.
Article21. Roozendaal C, Zhao MH, Horst G, Lockwood CM, Kleibeuker JH, Limburg PC, Nelis GF, Kallenberg CG. Catalase and alpha-enolase: two novel granulocyte autoantigens in inflammatory bowel disease (IBD). Clin Exp Immunol. 1998; 112:10–16. PMID: 9566783.22. Royds JA, Timperley WR, Taylor CB. Levels of enolase and other enzymes in the cerebrospinal fluid as indices of pathological change. J Neurol Neurosurg Psychiatry. 1981; 44:1129–1135. PMID: 7334408.
Article23. Roine RO, Somer H, Kaste M, Viinikka L, Karonen SL. Neurological outcome after out-of-hospital cardiac arrest. Prediction by cerebrospinal fluid enzyme analysis. Arch Neurol. 1989; 46:753–756. PMID: 2742544.24. Hay E, Royds JA, Davies-Jones GA, Lewtas NA, Timperley WR, Taylor CB. Cerebrospinal fluid enolase in stroke. J Neurol Neurosurg Psychiatry. 1984; 47:724–729. PMID: 6747647.
Article25. Grigoriadis G, Anderson MA, Whitehead S. Charcot-Leyden crystals. Intern Med J. 2010; 40:792. PMID: 21155159.26. Di Sabatino A, Battista N, Biancheri P, Rapino C, Rovedatti L, Astarita G, Vanoli A, Dainese E, Guerci M, Piomelli D, Pender SL, MacDonald TT, Maccarrone M, Corazza GR. The endogenous cannabinoid system in the gut of patients with inflammatory bowel disease. Mucosal Immunol. 2011; 4:574–583. PMID: 21471961.
Article27. Ackerman SJ, Liu L, Kwatia MA, Savage MP, Leonidas DD, Swaminathan GJ, Acharya KR. Charcot-Leyden crystal protein (galectin-10) is not a dual function galectin with lysophospholipase activity but binds a lysophospholipase inhibitor in a novel structural fashion. J Biol Chem. 2002; 277:14859–14868. PMID: 11834744.
Article28. Lin TA, Kourteva G, Hilton H, Li H, Tare NS, Carvajal V, Hang JS, Wei X, Renzetti LM. The mRNA level of Charcot-Leyden crystal protein/galectin-10 is a marker for CRTH2 activation in human whole blood in vitro. Biomarkers. 2010; 15:646–654. PMID: 20858065.29. Fujita M, Ichinose S, Kiyono T, Tsurumi T, Omori A. Establishment of latrunculin-A resistance in HeLa cells by expression of R183A D184A mutant beta-actin. Oncogene. 2003; 22:627–631. PMID: 12555075.30. Nunoi H, Yamazaki T, Tsuchiya H, Kato S, Malech HL, Matsuda I, Kanegasaki S. A heterozygous mutation of beta-actin associated with neutrophil dysfunction and recurrent infection. Proc Natl Acad Sci USA. 1999; 96:8693–8698. PMID: 10411937.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Advances in ulcerative colitis therapy
- Malignant change of chronic ulcerative colitis : report of a case
- Usefulness of Magnifying Chromoscopy in Ulcerative Colitis
- Alternation of mucin structure and lectin binding in the mucosa of ulcerative colitis
- Clinicopathologic Characteristics of Ulcerative Colitis Diagnosed by Endoscopic Biopsy Specimen: An analysis of discrepancy between clinical and pathologic diagnosis