Yonsei Med J.
2009 Jun;50(3):399-406. 10.3349/ymj.2009.50.3.399.
Expression of Heat Shock Protein 60 kDa Is Upregulated in Cervical Cancer
- Affiliations
-
- 1Division of Biological Science, Gachon University of Medicine and Science, Incheon, Korea. davekim@gachon.ac.kr
- 2Department of Obstetrics and Gynecology, Gachon University of Medicine and Science, Incheon, Korea.
- 3Department of Pharmacology, Korea University College of Medicine, Seoul, Korea.
- KMID: 1758569
- DOI: http://doi.org/10.3349/ymj.2009.50.3.399
Abstract
-
PURPOSE: Cervical cancer caused by the human papilloma virus (HPV) continues to be the cause of yearly death among women. However, it is a curable disease when diagnosed at an early stage. Recently, several researches have reported that heat shock protein (HSP) 60, a chaperone protein of molecular weight of 60 kDa, is involved in carcinogenesis and apoptosis. In order to evaluate the prognostic significance of HSP60 in cervical cancer, we examined differences in the HSP60 expression between cervical cancer and normal tissues in women.
MATERIALS AND METHODS
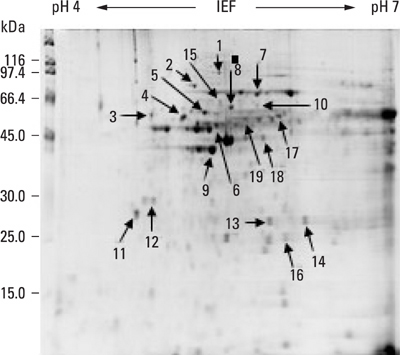
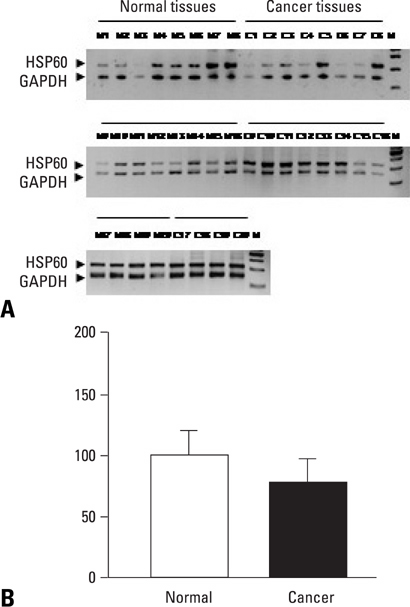
Tissue samples were collected from 20 cervical cancer patients and 20 normal controls. HSP60 expression of cervical cancer and normal tissues were verified by the 2D gel proteomics, semi-quantitative reverse transcriptase polymerase chain reaction (RT-PCR) and Western blot analyses.
RESULTS
In 2D proteomic analysis, an increase of HSP60 expression was detected in cervical cancer tissues and confirmed by Western blot analysis (p < 0.05). However, messenger RNA (mRNA) levels of HSP60 did not display any significant differences between cervical cancer and normal tissues.
CONCLUSION
These results suggest that HSP60 may be involved in the development of cervical cancer and have profound biological and prognostic significance.
MeSH Terms
Figure
Reference
-
1. Bosch FX, Manos MM, Muhoz N, Sherman M, Jansen AM, Peto J, et al. International biological study on cervical cancer (IBSCC) Study Group. Prevalence of human papillomavirus in cervical cancer: a worldwide perspective. J Natl Cancer Inst. 1995. 87:796–802.2. Walboomers JM, Jacobs MV, Manos MM, Bosch FX, Kummer JA, Shah KV, et al. Human papillomavirus is a necessary cause of invasive cervical cancer worldwide. J Pathol. 1999. 189:12–19.3. Munoz N, Bosch FX, de Sanjosé S, Herrero R, Castellsagué X, Shah KV, et al. Epidemiologic classification of human papillomavirus types associated with cervical cancer. N Engl J Med. 2003. 348:518–527.
Article4. Klaes R, Woerner SM, Ridder R, Wentzensen N, Duerst M, Schneider A, et al. Detection of high-risk cervical intraepithelial neoplasia and cervical cancer by amplification of transcripts derived from integrated papillomavirus oncogenes. Cancer Res. 1999. 59:6132–6136.5. Furumoto H, Irahara M. Human papilloma virus (HPV) and cervical cancer. J Med Invest. 2002. 49:124–133.6. Charvet I, Meda P, Genet M, Pelte MF, Vlastos AT. [Optical diagnosis of cervical dysplasia]. Bull Cancer. 2004. 91:45–53.7. Czarnecka AM, Campanella C, Zummo G, Cappello F. Mitochondrial chaperones in cancer: from molecular biology to clinical diagnostics. Cancer Biol Ther. 2006. 5:714–720.
Article8. Höhfeld J, Cyr DM, Patterson C. From the cradle to the grave: molecular chaperones that may choose between folding and degradation. EMBO Rep. 2001. 2:885–890.
Article9. Eng C, Kiuru M, Fernandez MJ, Aaltonen LA. A role for mitochondrial enzymes in inherited neoplasia and beyond. Nat Rev Cancer. 2003. 3:193–202.
Article10. Srivastava PK, Maki RG. Stress-induced proteins in immune response to cancer. Curr Top Microbiol Immunol. 1991. 167:109–123.
Article11. Ellis RJ. Molecular chaperones: pathways and networks. Curr Biol. 1999. 9:R137–R139.12. Jolly C, Morimoto RI. Role of the heat shock response and molecular chaperones in oncogenesis and cell death. J Natl Cancer Inst. 2000. 92:1564–1572.
Article13. Soltys BJ, Gupta RS. Mitochondrial-matrix proteins at unexpected locations: are they exported? Trends Biochem Sci. 1999. 24:174–177.
Article14. Hansen JJ, Bross P, Westergaard M, Nielsen MN, Eiberg H, Børglum AD, et al. Genomic structure of the human mitochondrial chaperonin genes: HSP60 and HSP10 are localised head to head on chromosome 2 separated by a bidirectional promoter. Hum Genet. 2003. 112:71–77.15. Samali A, Cai J, Zhivotovsky B, Jones DP, Orrenius S. Presence of a pre-apototic complex of pro-caspase-3, Hsp60 and Hsp10 in the mitochondrial fraction of jurkat cells. EMBO J. 1999. 18:2040–2048.16. Bini L, Magi B, Marzocchi B, Arcuri F, Tripodi S, Cintorino M, et al. Protein expression profiles in human breast ductal carcinoma and histologically normal tissue. Electrophoresis. 1997. 18:2832–2841.
Article17. Schneidér J, Jimenez E, Marenbach K, Romero H, Mark D, Meden H. Immunohistochemical detection of HSP60-expression in human ovarian cancer. Correlation with survival in a series of 247 patients. Anticancer Res. 1999. 19:2141–2146.18. Cappello F, Bellafiore M, Palma A, Marciano V, Martorana G, Belfiore P, et al. Expression of 60-kD heat shock protein increases during carcinogenesis in the uterine exocervix. Pathobiology. 2002-2003. 70:83–88.
Article19. Cappello F, Bellafiore M, Palma A, David S, Marcianó V, Bartolotta T, et al. 60KDa chaperonin (HSP60) is over-expressed during colorectal carcinogenesis. Eur J Histochem. 2003. 47:105–110.
Article20. Cappello F, Rappa F, David S, Anzalone R, Zummo G. Immunohistochemical evaluation of PCNA, p53, HSP60, HSP10 and MUC-2 presence and expression in prostate carcinogenesis. Anticancer Res. 2003. 23:1325–1331.21. Pignatelli D, Ferreira J, Soares P, Costa MJ, Magalhaes MC. Immunohistochemical study of heat shock proteins 27, 60 and 70 in the normal human adrenal and in adrenal tumors with suppressed ACTH production. Microsc Res Tech. 2003. 61:315–323.
Article22. Cappello F, David S, Rappa F, Bucchieri F, Marasá L, Bartolotta TE, et al. The expression of HSP60 and HSP10 in large bowel carcinomas with lymph node metastase. BMC Cancer. 2005. 5:139.
Article23. Cappello F, Di Stefano A, D'Anna SE, Donner CF, Zummo G. Immunopositivity of heat shock protein 60 as a biomarker of bronchial carcinogenesis. Lancet Oncol. 2005. 6:816.
Article24. Castle PE, Ashfaq R, Ansari F, Muller CY. Immunohistochemical evaluation of heat shock proteins in normal and preinvasive lesions of the cervix. Cancer Lett. 2005. 229:245–252.
Article25. Kim JY, Shin HJ, Kim TH, Cho KH, Shin KH, Kim BK, et al. Tumor-associated carbonic anhydrases are linked to metastases in primary cervical cancer. J Cancer Res Clin Oncol. 2006. 132:302–308.
Article26. Matsui NM, Smith-Beckerman DM, Epstein LB. Staining of preparative 2-D gels. Coomassie blue and imidazole-zinc negative staining. Methods Mol Biol. 1999. 112:307–311.27. Cornford PA, Dodson AR, Parsons KF, Desmond AD, Woolfenden A, Fordham M, et al. Heat Shock Protein Expression Independently predicts clinical outcome in prostate cancer. Cancer Res. 2000. 60:7099–7105.28. Kirchhoff SR, Gupta S, Knowlton AA. Cytosolic heat shock protein 60, apoptosis, and myocardial injury. Circulation. 2002. 105:2899–2904.
Article29. Cappello F, Di Stefano A, David S, Rappa F, Anzalone R, La Rocca G, et al. Hsp60 and Hsp10 down-regulation predicts bronchial epithelial carcinogenesis in smokers with chronic obstructive pulmonary disease. Cancer. 2006. 107:2417–2424.
Article30. Kimura E, Enns RE, Alcaraz JE, Arboleda J, Slamon DJ, Howell SB. Correlation of the survival of ovarian cancer patients with mRNA expression of the 60-kD heat-shock protein HSP-60. J Clin Oncol. 1993. 11:891–898.31. Chant ID, Rose PE, Morris AG. Analysis of heat-shock protein expression in myeloid leukemia cells by flow cytometry. Br J Haematol. 1995. 90:163–168.
Article32. Franzén B, Linder S, Alaiya AA, Eriksson E, Fujioka K, Berman AC, et al. Analysis of polypeptide expression in benign and malignant human breast lesions. Electrophoresis. 1997. 18:582–587.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Environmental factors regulating the expression of Porphyromonas gingivalis heat shock protein
- Expression of Heat Shock Protein 70 m-RNA in Rat Bladder Overdistended by Diuresis
- The Role of Inducible 70-kDa Heat Shock Protein in Cell Cycle Control, Differentiation, and Apoptotic Cell Death of the Human Myeloid Leukemic HL-60 Cells
- The Protective Effect of Induced Heat Shock Protein in Human Corneal Epithelial Cells
- The Expression of Heat Shock Protein 60 kDa in Tissues and Cell Lines of Breast Cancer