J Korean Med Sci.
2014 Mar;29(3):338-342. 10.3346/jkms.2014.29.3.338.
Nomogram for Prediction of Prostate Cancer with Serum Prostate Specific Antigen Less than 10 ng/mL
- Affiliations
-
- 1Department of Urology and Biomedical Research Institute, Pusan National University Hospital, Busan, Korea. hongkooha@naver.com
- KMID: 1734919
- DOI: http://doi.org/10.3346/jkms.2014.29.3.338
Abstract
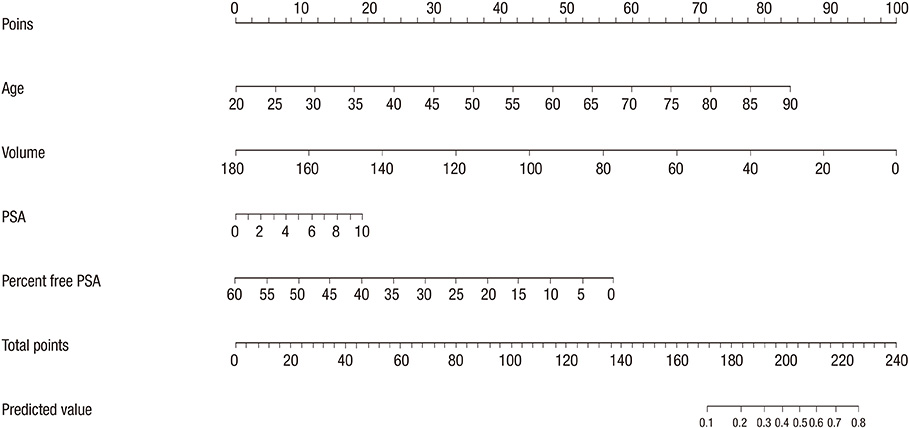
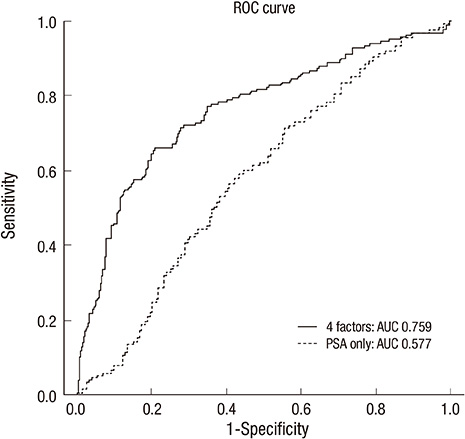
- Although prostate-specific antigen (PSA) is a very useful screening tool, prostate biopsy is still necessary to confirm prostate cancer (PCA). However, it is reported that PSA is associated with a high false-positive rate and prostate biopsy also has various procedure-related complications. Therefore, the authors have devised a nomogram, which can be used to estimate the risk of PCA, using available clinical data for men with a serum PSA less than 10 ng/mL. Prostate biopsies were obtained from 2,139 patients from January 1998 to March 2011. Of them, 1,171 patients with a serum PSA less than 10 ng/mL were only included in this study. Patient age, PSA, free PSA, prostate volume, PSA density and percent free PSA ratio were analyzed. Among 1,171 patients, 255 patients (21.8%) were diagnosed as PCA. Multivariate analyses showed that patient age, prostate volume, PSA and percent free PSA had statistically significant relationships with PCA (P < 0.05) and were used as nomogram predictor variables. The area under the (ROC) curve for all factors in a model predicting PCA was 0.759 (95% CI, 0.716-0.803).
Keyword
MeSH Terms
Figure
Cited by 1 articles
-
Visually Estimated MRI Targeted Prostate Biopsy Could Improve the Detection of Significant Prostate Cancer in Patients with a PSA Level <10 ng/mL
Dong Hoon Lee, Jong Kil Nam, Sung Woo Park, Seung Soo Lee, Ji-Yeon Han, Sang Don Lee, Joon Woo Lee, Moon Kee Chung
Yonsei Med J. 2016;57(3):565-571. doi: 10.3349/ymj.2016.57.3.565.
Reference
-
1. American Cancer Society. Cancer facts and figures 2008. accessed on 15 February 2013. Available at http://www.cancer.org/acs/groups/content/@nho/documents/document/2008cafffinalsecuredpdf.pdf.2. Boyle P, Ferlay J. Cancer incidence and mortality in Europe, 2004. Ann Oncol. 2005; 16:481–488.3. National Cancer Center. Cancer facts and figures 2011. accessed on 15 February 2013. Available at http://www.cancer.go.kr/ncic/cics_g/cics_g02/cics_g027/1647906_6065.html.4. Andriole GL, Crawford ED, Grubb RL 3rd, Buys SS, Chia D, Church TR, Fouad MN, Gelmann EP, Kvale PA, Reding DJ, et al. Mortality results from a randomized prostate-cancer screening trial. N Engl J Med. 2009; 360:1310–1319.5. Catalona WJ, Smith DS, Ratliff TL, Dodds KM, Coplen DE, Yuan JJ, Petros JA, Andriole GL. Measurement of prostate-specific antigen in serum as a screening test for prostate cancer. N Engl J Med. 1991; 324:1156–1161.6. Schröder FH, Hugosson J, Roobol MJ, Tammela TL, Ciatto S, Nelen V, Kwiatkowski M, Lujan M, Lilja H, Zappa M, et al. Screening and prostate-cancer mortality in a randomized European study. N Engl J Med. 2009; 360:1320–1328.7. Thompson IM, Pauler DK, Goodman PJ, Tangen CM, Lucia MS, Parnes HL, Minasian LM, Ford LG, Lippman SM, Crawford ED, et al. Prevalence of prostate cancer among men with a prostate-specific antigen level < or =4.0 ng per milliliter. N Engl J Med. 2004; 350:2239–2246.8. Chun FK, Epstein JI, Ficarra V, Freedland SJ, Montironi R, Montorsi F, Shariat SF, Schröder FH, Scattoni V. Optimizing performance and interpretation of prostate biopsy: a critical analysis of the literature. Eur Urol. 2010; 58:851–864.9. Wagenlehner FM, van Oostrum E, Tenke P, Tandogdu Z, Çek M, Grabe M, Wullt B, Pickard R, Naber KG, Pilatz A, et al. Infective complications after prostate biopsy: outcome of the Global Prevalence Study of Infections in Urology (GPIU) 2010 and 2011, a prospective multinational multicentre prostate biopsy study. Eur Urol. 2013; 63:521–527.10. Gancarczyk KJ, Wu H, McLeod DG, Kane C, Kusuda L, Lance R, Herring J, Foley J, Baldwin D, Bishoff JT, et al. Using the percentage of biopsy cores positive for cancer, pretreatment PSA, and highest biopsy Gleason sum to predict pathologic stage after radical prostatectomy: the Center for Prostate Disease Research nomograms. Urology. 2003; 61:589–595.11. Ilic D, O'Connor D, Green S, Wilt T. Screening for prostate cancer: a Cochrane systematic review. Cancer Causes Control. 2007; 18:279–285.12. Wang MC, Valenzuela LA, Murphy GP, Chu TM. Purification of a human prostate specific antigen. Invest Urol. 1979; 17:159–163.13. Ferro MA, Barnes I, Roberts JB, Smith PJ. Tumour markers in prostatic carcinoma: a comparison of prostate-specific antigen with acid phosphatase. Br J Urol. 1987; 60:69–73.14. Loeb S, Carter HB, Berndt SI, Ricker W, Schaeffer EM. Complications after prostate biopsy: data from SEER-Medicare. J Urol. 2011; 186:1830–1834.15. Loeb S, van den Heuvel S, Zhu X, Bangma CH, Schröder FH, Roobol MJ. Infectious complications and hospital admissions after prostate biopsy in a European randomized trial. Eur Urol. 2012; 61:1110–1114.16. Nam RK, Saskin R, Lee Y, Liu Y, Law C, Klotz LH, Loblaw DA, Trachtenberg J, Stanimirovic A, Simor AE, et al. Increasing hospital admission rates for urological complications after transrectal ultrasound guided prostate biopsy. J Urol. 2010; 183:963–968.17. Kawamura K, Suzuki H, Kamiya N, Imamoto T, Yano M, Miura J, Shimbo M, Suzuki N, Nakatsu H, Ichikawa T. Development of a new nomogram for predicting the probability of a positive initial prostate biopsy in Japanese patients with serum PSA levels less than 10 ng/mL. Int J Urol. 2008; 15:598–603.18. Carlson GD, Calvanese CB, Partin AW. An algorithm combining age, total prostate-specific antigen (PSA), and percent free PSA to predict prostate cancer: results on 4298 cases. Urology. 1998; 52:455–461.19. Eastham JA, May R, Robertson JL, Sartor O, Kattan MW. Development of a nomogram that predicts the probability of a positive prostate biopsy in men with an abnormal digital rectal examination and a prostate-specific antigen between 0 and 4 ng/mL. Urology. 1999; 54:709–713.20. Karakiewicz PI, Benayoun S, Kattan MW, Perrotte P, Valiquette L, Scardino PT, Cagiannos I, Heinzer H, Tanguay S, Aprikian AG, et al. Development and validation of a nomogram predicting the outcome of prostate biopsy based on patient age, digital rectal examination and serum prostate specific antigen. J Urol. 2005; 173:1930–1934.21. Optenberg SA, Clark JY, Brawer MK, Thompson IM, Stein CR, Friedrichs P. Development of a decision-making tool to predict risk of prostate cancer: the Cancer of the Prostate Risk Index (CAPRI) test. Urology. 1997; 50:665–672.22. Zaytoun OM, Kattan MW, Moussa AS, Li J, Yu C, Jones JS. Development of improved nomogram for prediction of outcome of initial prostate biopsy using readily available clinical information. Urology. 2011; 78:392–398.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Prostate specific antigen as a tumor marker for adenocarcinoma of the prostate
- Significance of Serum Testosterone for Prostate-Specific Antigen (PSA) Elevation and Prediction of Prostate Cancer in Patients with PSA Above 10 ng/ml
- Detection Rate of Prostate Cancer on the Basis of the Vienna Nomogram: A Singapore Study
- Nomogram Using Prostate Health Index for Predicting Prostate Cancer in the Gray Zone: Prospective, Multicenter Study
- Effects of Medication with Dutasteride on Detection of Prostate Cancer in Patients with Serum Prostate-specific Antigen Level of 4~10 ng/ml