J Korean Med Sci.
2004 Apr;19(2):245-252. 10.3346/jkms.2004.19.2.245.
Regulation of Glomerular Endothelial Cell Proteoglycans by Glucose
- Affiliations
-
- 1Department of Pediatrics, Chungbuk National University, Cheongju, Korea. tsha@med.chungbuk.ac.kr
- 2Department of Medicine, University of Texas Health Science Center, South Texas Veteran's Health Care System, San Antonio, Texas, U.S.A.
- KMID: 1733484
- DOI: http://doi.org/10.3346/jkms.2004.19.2.245
Abstract
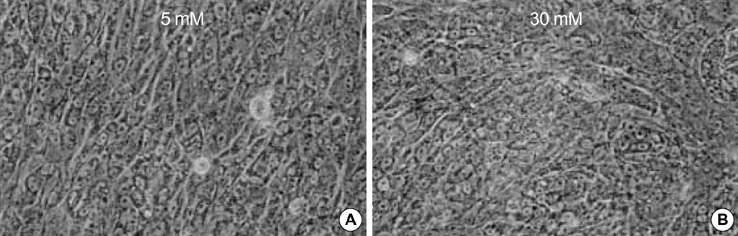
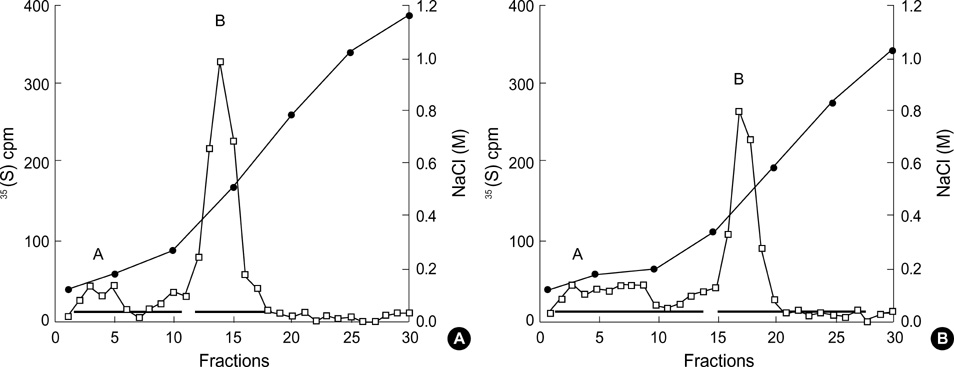
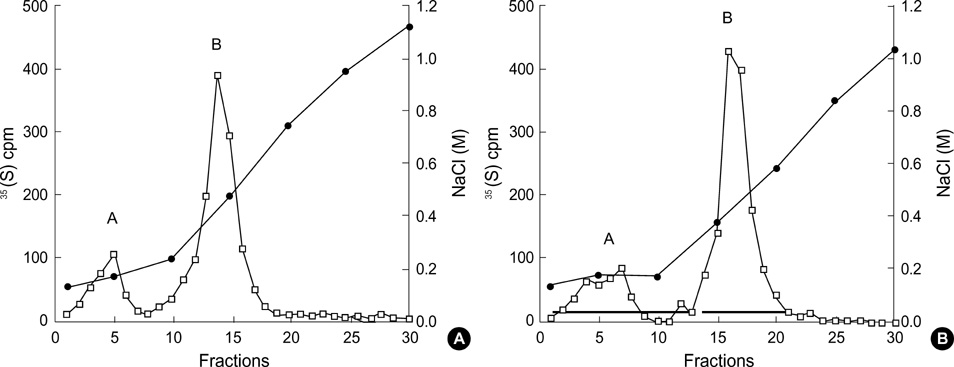
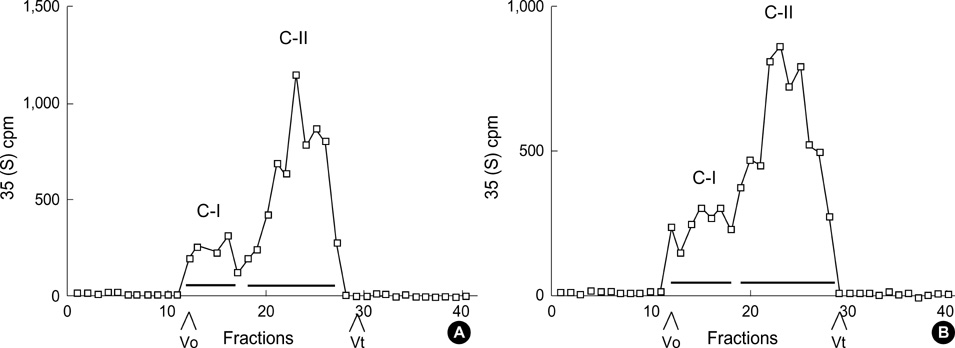
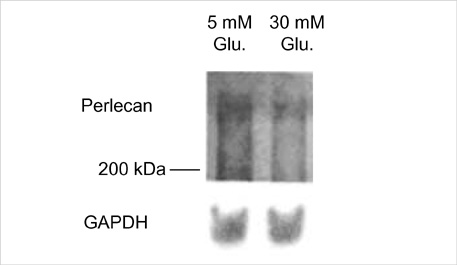
- The presence of heparan sulfate proteoglycan (HSPG) in anionic sites in the lamina rara interna of glomerular basement membrane suggests that the proteoglycan may be deposited by the glomerular endothelial cells (GEndo). We have previously demonstrated that bovine GEndo in vitro synthesize perlecan, a species of glomerular basement membrane HSPG. In this study we examined whether high glucose medium regulates the GEndo metabolism of glycopeptides including perlecan. Metabolic labeling of glycoconjugates with 35S-SO4, sequential ion exchange and Sepharose CL-4B chromatography of labeled glycoconjugates, and northern analysis were performed. Incubation of GEndo for 8 to 14 weeks (but not for 1-2 weeks) in medium containing 30 mM glucose resulted in nearly 50% reduction in the synthesis of cell layer and medium 35SO4-labeled low anionic glycoproteins and proteoglycans, including that of basement membrane HSPG (Kav 0.42) compared to GEndo grown in 5 mM glucose medium; no changes in anionic charge density or hydrodynamic size of proteoglycans were noted. Northern analysis demonstrated that the mRNA abundance of perlecan was reduced by 47% in cells incubated with 30 mM glucose. Our data suggest that high glucose medium reduces the GEndo synthesis of perlecan by regulating its gene expression. Reduced synthesis of perlecan by GEndo may contribute to proteinuria seen in diabetic nephropathy.
Keyword
MeSH Terms
-
Animals
Basement Membrane/drug effects/metabolism
Cattle
Cells, Cultured
Diabetic Nephropathies/metabolism
Endothelial Cells/cytology/*drug effects/*metabolism
Gene Expression/drug effects
Glucose/*pharmacology
Heparan Sulfate Proteoglycan/genetics/*metabolism
Kidney Glomerulus/*cytology
Sulfur Radioisotopes/diagnostic use
Support, Non-U.S. Gov't
Support, U.S. Gov't, Non-P.H.S.
Support, U.S. Gov't, P.H.S.
Figure
Reference
-
1. Vernier RL, Steffes MW, Sisson-Ross S, Mauer SM. Heparan sulfate proteoglycan in the glomerular basement membrane of type I diabetes mellitus. Kidney Int. 1992. 41:1070–1080.2. Kanwar YS, Linker A, Farquhar MG. Increased permeability of the glomerular basement membrane to ferritin after removal of glycosaminoglycans (heparan sulfate) by enzyme digestion. J Cell Biol. 1980. 86:688–693.
Article3. Rosenzweig LJ, Kanwar YS. Removal of sulfated (heparan sulfate) and nonsulfated (hyaluronic acid) glycosaminoglycans results in increased permeability of the glomerular basement membrane to 125I-bovine serum albumin. Lab Invest. 1982. 47:177–184.4. Kasinath BS, Kanwar YS. Rohrbach DH, Timpl R, editors. Glomerular Basement Membrane: Biology and Physiology. Molecular and Cellular Aspects of Basement Membrane. 1993. San Diego: Academic Press;89–106.
Article5. Stow JL, Sawada H, Farquhar MG. Basement membrane heparan sulfate proteoglycans are concentrated in the laminae rarae and in podocytes of the rat renal glomerulus. Proc Natl Acad Sci USA. 1985. 82:3296–3300.
Article6. Thomas GJ, Jenner L, Mason RM, Davies M. Human glomerular epithelial cell proteoglycans. Arch Biochem Biophys. 1990. 278:11–20.
Article7. Klein DJ, Oegema TR Jr, Fredeen TS, van der Woude F, Kim Y, Brown DM. Partial characterization of proteoglycans synthesized by human glomerular epithelial cells in culture. Arch Biochem Biophys. 1990. 277:389–401.
Article8. Kasinath BS, Block JA, Singh AK, Terhune WC, Maldonado R, Davalath S, Kallgren MJ, Wanna L. Regulation of rat glomerular epithelial cell proteoglycans by high glucose medium. Arch Biochem Biophys. 1994. 309:149–159.9. Yard BA, Kahlert S, Engelleiter R, Resch S, Waldherr R, Groffen AJ, van den Heuvel LP, van der Born J, Berden JH, Kroger S, Hafner M, van dervWoude FJ. Decreased glomerular expression of agrin in diabetic nephropathy and podocytes, cultured in high glucose medium. Exp Nephrol. 2001. 9:214–222.
Article10. Kasinath BS, Grellier P, Choudhury GG, Abboud SL. Regulation of basement membrane heparan sulfate proteoglycan, perlecan, gene expression in glomerular epithelial cells by high glucose medium. J Cell Physiol. 1996. 167:131–136.
Article11. Iozzo RV, Murdoch AD. Proteoglycans of the extracellular environment: clues from the gene and protein side offer novel perspectives in molecular diversity and function. FASEB J. 1996. 10:598–614.
Article12. Kasinath BS. Glomerular endothelial cell proteoglycans: Regulation by TGFβ-1. Arch Biochem Biophys. 1993. 305:370–377.13. Kasinath BS. Effect of insulin on high glucose medium induced changes in rat glomerular epithelial cell metabolism of glycoconjugates. Arch Biochem Biophys. 1995. 318:286–294.14. Yanagishita M, Midura RJ, Hascall VC. Proteoglycans: Isolation and purification from tissue cultures. Methods Enzymol. 1987. 138:279–289.15. Ko CW, Bhandari B, Yee J, Terhune WC, Maldonado R, Kasinath BS. Cyclic AMP regulates basement membrane heparan sulfate proteoglycan, perlecan, metabolism in rat glomerular epithelial cells. Mol Cell Biochem. 1996. 162:65–73.
Article16. Parthasarathy N, Spiro RG. Isolation and characterization of the heparan sulfate proteoglycan of the bovine glomerular basement membrane. J Biol Chem. 1984. 259:12749–12755.
Article17. Abrahamson DR. Origin of the glomerular basement membrane visualized after in vivo labeling of laminin in newborn rat kidneys. J Cell Biol. 1981. 100:1988–2000.
Article18. Young BA, Johnson RJ, Alpers CE, Eng E, Gordon K, Floege J, Couser WG, Seidel K. Cellular events in the evolution of experimental diabetic nephropathy. Kidney Int. 1995. 47:935–944.
Article19. Voziyan PA, Khalifah RG, Thibaudeau C, Yildiz A, Jacob J, Serianni AS, Hudson BG. Modification of proteins in vitro by physiological levels of glucose: pyridoxamine inhibits conversion of Amadori intermediate to advanced glycation end-products through binding of redox metal ions. J Biol Chem. 2003. 278:46616–46624.20. Ha T-S, Kim H-S. Effects of advanced glycation endproducts on rat glomerular epithelial cells: Roles of reactive oxygen species. Korean J Nephrol. 2003. 22:285–293.21. Thompson KS, Towle HC. Localization of the carbohydrate response element of the rat L-type pyruvate kinase gene. J Biol Chem. 1991. 266:8679–8682.
Article22. Shin HM, Towle HC. Definition of carbohydrate response element of the rat S14 gene. Evidence for a common factor required for carbohydrate regulation of hepatic genes. J Biol Chem. 1992. 267:13222–13228.23. Vaulont S, Kahn A. Transcriptional control of metabolic regulation genes by carbohydrates. FASEB J. 1994. 8:28–35.
Article24. Carlin B, Jaffe R, Bender B, Chung AE. Entactin, a novel basal lamina-associated glycoprotein. J Biol Chem. 1981. 256:5209–5214.25. Timpl R, Dziadek M, Fujiwara S, Nowack H, Wick G. Nidogen: a new self-aggregating basement membrane protein. Eur J Biochem. 1983. 137:455–465.
Article26. Katz A, Fish AJ, Kleppel MM, Hagen SG, Michael AF, Butkowski RJ. Renal entactin (nidogen): isolation, characterization and tissue distribution. Kidney Int. 1991. 40:643–652.
Article27. Hogan BL, Taylor A, Kurkinen M, Couchman JR. Synthesis and localization of two sulphated glycoproteins associated with basement membranes and the extracellular matrix. J Cell Biol. 1982. 95:197–204.
Article28. Cohen MP, Wu VY, Surma ML. Non-collagen protein and proteoglycan in renal glomerular basement membrane. Biochem Biophys Acta. 1981. 678:322–328.
Article29. Lemkin MC, Farquhar MG. Sulfated and nonsulfated glycosaminoglycans and glycopeptides are synthesized by kidney in vivo and incorporated into glomerular basement membranes. Proc Natl Acad Sci USA. 1981. 78:1726–1730.
Article30. Ha T-S, Barnes JL, Stewart JL, Ko CW, Miner JH, Abrahamson DR, Sanes JR, Kasinath BS. Regulation of renal laminin in mice with type II diabetes. J Am Soc Nephrol. 1999. 10:1931–1939.
Article31. Tilton RG, Chang K, Pugliese G, Eades DM, Province MA, Sherman WR, Kilo C, Williamson JR. Prevention of hemodynamic and vascular albumin filtration changes in diabetic rats by aldose reductase inhibitors. Diabetes. 1989. 38:1258–1270.
Article32. Pugliese G, Tilton RG, Speedy A, Santarelli E, Eades DM, Province MA, Kilo C, Sherman WR, Williamson JR. Modulation of hemodynamic and vascular filtration changes in diabetic rats by dietary myo-inositol. Diabetes. 1990. 39:312–322.
Article33. Vlassara H, Bucala R, Striker L. Pathogenic effects of advanced glycosylation: biochemical, biological and clinical implications for diabetes and aging. Lab Invest. 1993. 70:138–151.34. Ziyadeh FN, Sharma K, Ericksen M, Wolf G. Stimulation of collagen gene expression and protein synthesis in murine mesangial cells by high glucose is mediated by autocrine activation of transforming growth factor-β. J Clin Invest. 1994. 93:536–542.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Glomerular Basement Membrane Heparan Sulfate Proteoglycan (GBM HSPG)
- High glucose stimulates the expression of erythropoietin in rat glomerular epithelial cells
- Culture of the Human Glomerular Endothelial Cells
- The Effect of Cyclic AMP on Gene Regulation of Glomerular Basement Membrane Heparan Sulfate Proteoglycan in Rat Glomerular Epithelial Cells
- Endothelial cell autophagy in the context of disease development