J Korean Med Sci.
2004 Apr;19(2):202-208. 10.3346/jkms.2004.19.2.202.
Effect of Weight Reduction on Metabolic Syndrome in Korean Obese Patients
- Affiliations
-
- 1Department of Family Medicine, Asan Medical Center, University of Ulsan College of Medicine, Seoul, Korea. hyesoon@amc.seoul.kr
- 2Department of Neurosurgery, Korea University Medical Center, Seoul, Korea.
- KMID: 1733477
- DOI: http://doi.org/10.3346/jkms.2004.19.2.202
Abstract
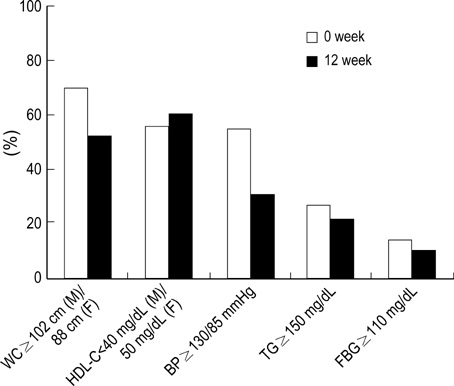
- The Third Report of the National Cholesterol Education Program Adult Treatment Panel III emphasized the importance of management of the metabolic syndrome. However, little information is available about the effect of weight reduction on the metabolic syndrome in obese patients among Koreans. A longitudinal clinical intervention study from the 12-week of weight reduction program, including life style modification and adjuvant appetite suppressants, in 78 obese persons was performed. Anthropometry and metabolic risk factors were measured before and after weight reduction. Visceral (VAT), subcutaneous (SAT), and total adipose tissue (TAT) on abdomen were determined by CT scan. Moderate decrease in weight (9.3%) induced significant reduction of waist circumference, systolic and diastolic blood pressure, and triglyceride. Weight reduction also resulted in significant decrease in total cholesterol, LDL-C, uric acid, fasting insulin, and HOMA score. The subjects with metabolic syndrome showed more improvements of metabolic components than those without metabolic syndrome through weight reduction. The reductions of visceral-subcutaneous fat ratio (VSR) and waist circumference were observed as for the predictable variables related to the improvement of metabolic component and insulin resistance in Korean obese patients.
Keyword
MeSH Terms
Figure
Reference
-
1. NHLBI. Executive Summary of The Third Report of The National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, And Treatment of High Blood Cholesterol In Adults (Adult Treatment Panel III). JAMA. 2001. 285:2486–2497.2. Lakka HM, Laaksonen DE, Lakka TA, Niskanen LK, Kumpusalo E, Tuomilehto J, Salonen JT. The metabolic syndrome and total and cardiovascular disease mortality in middle-aged men. JAMA. 2002. 288:2709–2716.
Article3. Isomaa B, Almgren P, Tuomi T, Forsen B, Lahti K, Nissen M, Taskinen MR, Groop L. Cardiovascular morbidity and mortality associated with the metabolic syndrome. Diabetes Care. 2001. 24:683–689.
Article4. Hauner H. Insulin resistance and the metabolic syndrome: a challenge of the new millennium. Eur J Clin Nutr. 2002. 56:Suppl 1. S25–S29.5. Hjermann I. The metabolic cardiovascular syndrome: syndrome X, Reaven's syndrome, insulin resistance syndrome, atherothrombogenic syndrome. J Cardiovasc Pharmacol. 1992. 20:Suppl 8. S5–S10.
Article6. Ohlson LO, Larsson B, Svardsudd K, Welin L, Eriksson H, Wilhelmsen L, Bjorntorp P, Tibblin G. The influence of body fat distribution on the incidence of diabetes mellitus. 13.5 years of follow-up of the participants in the study of men born in 1913. Diabetes. 1985. 34:1055–1058.
Article7. Masuda T, Imai K, Komiya S. Relationship of anthropometric indices of body fat to cardiovascular risk in Japanese women. Ann Physiol Anthropol. 1993. 12:135–144.
Article8. McKeigue PM, Shah B, Marmot MG. Relation of central obesity and insulin resistance with high diabetes prevalence and cardiovascular risk in South Asians. Lancet. 1991. 337:382–386.
Article9. Han TS, van Leer EM, Seidell JC, Lean ME. Waist circumference action levels in the identification of cardiovascular risk factors: prevalence study in a random sample. BMJ. 1995. 311:1401–1405.
Article10. Maison P, Byrne CD, Hales CN, Day NE, Wareham NJ. Do different dimensions of the metabolic syndrome change together over time? Evidence supporting obesity as the central feature. Diabetes Care. 2001. 24:1758–1763.
Article11. Korea National Statistical Office. 2000 Report of Statistics in Mortality in Korean. 2001.12. Park HS. Epidemiology of metabolic syndrome among South Koreans. Korean J Obes. 2002. 11:203–211.13. Cha K, Chertow GM, Gonzalez J, Lazarus JM, Wilmore DW. Multifrequency bioelectrical impedance estimates the distribution of body water. J Appl Physiol. 1995. 79:1316–1319.
Article14. Ferland M, Despres JP, Tremblay A, Pinault S, Nadeau A, Moorjani S, Lupien PJ, Theriault G, Bouchard C. Assessment of adipose tissue distribution by computed axial tomography in obese women: association with body density and anthropometric measurements. Br J Nutr. 1989. 61:139–148.
Article15. Sjostrom L, Kvist H, Cederblad A, Tylen U. Determination of total adipose tissue and body fat in women by computed tomography, 40K, and tritium. Am J Physiol. 1986. 250:E736–E745.
Article16. American Society of Hypertension. Recommendations for routine blood pressure measurement by indirect cuff sphygmomanometry. Am J Hypertens. 1992. 5:207–209.17. Friedewald WT, Levy RI, Fredrickson DS. Estimation of the concentration of low-density lipoprotein cholesterol in plasma, without use of the preparative ultracentrifuge. Clin Chem. 1972. 18:499–502.
Article18. Matthews DR, Hosker JP, Rudenski AS, Naylor BA, Treacher DF, Turner RC. Homeostasis model assessment: insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia. 1985. 28:412–419.19. Korean Nutrition Society. Computerized Aided Nutritional analysis program. 1998.20. Ainsworth BE, Haskell WL, Leon AS, Jacobs DR Jr, Montoye HJ, Sallis JF, Paffenbarger RS Jr. Compendium of physical activities: classification of energy costs of human physical activities. Med Sci Sports Exerc. 1993. 25:71–80.
Article21. Tuomilehto J, Lindstrom J, Eriksson JG, Valle TT, Hamalainen H, Ilanne-Parikka P, Keinanen-Kiukaanniemi S, Laakso M, Louheranta A, Rastas M, Salminen V, Uusitupa M; Finnish Diabetes Prevention Study Group. Prevention of type 2 diabetes mellitus by changes in lifestyle among subjects with impaired glucose tolerance. N Engl J Med. 2001. 344:1343–1350.
Article22. Knowler WC, Barrett-Connor E, Fowler SE, Hamman RF, Lachin JM, Walker EA, Nathan DM; Diabetes Prevention Program Research Group. Reduction in the incidence of type 2 diabetes with lifestyle intervention or metformin. N Engl J Med. 2002. 346:393–403.
Article23. Fujioka S, Matsuzawa Y, Tokunaga K, Kawamoto T, Kobatake T, Keno Y, Kotani K, Yoshida S, Tarui S. Improvement of glucose and lipid metabolism associated with selective reduction of intra-abdominal visceral fat in premenopausal women with visceral fat obesity. Int J Obes. 1991. 15:853–859.24. Zamboni M, Armellini F, Turcato E, Todesco T, Bissoli L, Bergamo-Andreis IA, Bosello O. Effect of weight loss on regional body fat distribution in premenopausal women. Am J Clin Nutr. 1993. 58:29–34.
Article25. Arner P. Differences in lipolysis between human subcutaneous and omental adipose tissues. Ann Med. 1995. 27:435–438.
Article26. International Obesity Task Force. Asia-Pacific perspective: redefining obesity and its treatment. 2000. Sydney: Western Pacific Region.27. Janssen I, Fortier A, Hudson R, Ross R. Effects of an energy-restrictive diet with or without exercise on abdominal fat, intermuscular fat, and metabolic risk factors in obese women. Diabetes Care. 2002. 25:431–438.
Article28. Wing RR, Jeffery RW. Effect of modest weight loss on changes in cardiovascular risk factors: are there differences between men and women or between weight loss and maintenance? Int J Obes Relat Metab Disord. 1995. 19:67–73.29. Dengel DR, Galecki AT, Hagberg JM, Pratley RE. The independent and combined effects of weight loss and aerobic exercise on blood pressure and oral glucose tolerance in older men. Am J Hypertens. 1998. 11:1405–1412.
Article30. Su HY, Sheu WH, Chin HM, Jeng CY, Chen YD, Reaven GM. Effect of weight loss on blood pressure and insulin resistance in normotensive and hypertensive obese individuals. Am J Hypertens. 1995. 8:1067–1071.
Article31. McLaughlin T, Abbasi F, Carantoni M, Schaaf P, Reaven G. Differences in insulin resistance do not predict weight loss in response to hypocaloric diets in healthy obese women. J Clin Endocrinol Metab. 1999. 84:578–581.
Article32. Alberti KG, Zimmet PZ. Definition, diagnosis and classification of diabetes mellitus and its complications. Part 1: diagnosis and classification of diabetes mellitus provisional report of a WHO consultation. Diabet Med. 1998. 15:539–553.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Effect of Orlistat on the Metabolic Syndrome in Obese Patients
- Association between Body Weight Perception and Metabolic Syndrome in Korean Adults: The Korea National Health and Nutrition Examination Survey 2015–2016
- The Impact of Weight Changes on Metabolic Syndrome over a Time period of 8 years in Korean Male Workers
- Effect of Weight Reduction on Obesity-specific Quality of Life (QOL) in Obese Subjects
- Clinical Predictive Factors for Metabolic Syndrome in Obese Children and Adolescents