Chonnam Med J.
2010 Aug;46(2):67-73. 10.4068/cmj.2010.46.2.67.
Sirt1 as a New Therapeutic Target in Metabolic and Age-Related Diseases
- Affiliations
-
- 1Department of Internal Medicine, Jeju National University School of Medicine, Jeju, Korea. Ldhkso@jejunu.ac.kr
- KMID: 1730001
- DOI: http://doi.org/10.4068/cmj.2010.46.2.67
Abstract
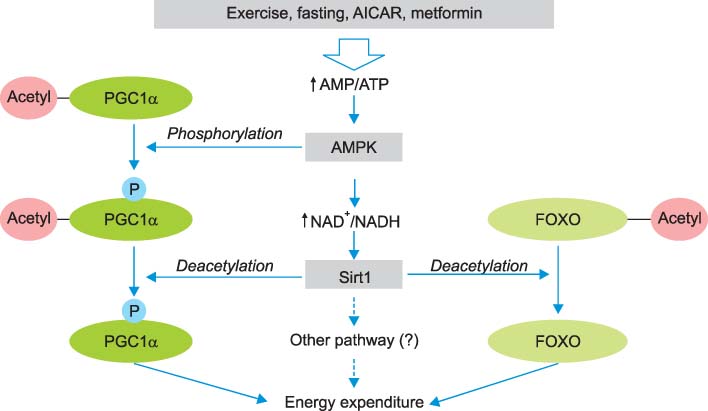
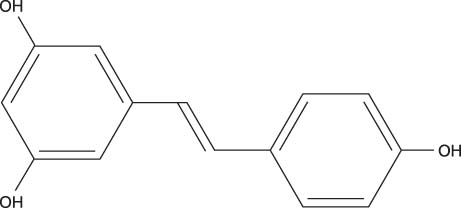
- Sirt1 among the mammalian sirtuins has generated intense scientific interest in recent years, mainly because of its effects on longevity, metabolism, and other aging-related processes. Via nicotinamide adenine dinucleotide (NAD)-dependent protein deacetylation, Sirt1 can regulate important metabolic regulators. Sirt1 is involved in insulin secretion in the pancreas, fatty acid oxidation in liver and skeletal muscle, hepatic gluconeogenesis during fasting, the suppression of fat storage in white adipose tissue, and the enhancement of insulin sensitivity in skeletal muscle. It also communicates with AMP-activated protein kinase (AMPK). Resveratrol, a prototype of Sirt1 activator, can induce similar changes reminiscent of caloric restriction in insulin-resistant obese rodent models, although clinical studies are still limited. Intensive research efforts are now targeting Sirt1 for pharmacologic interventions in aging and age-related metabolic and degenerative disorders.
MeSH Terms
Figure
Reference
-
1. Knutson MD, Leeuwenburgh C. Resveratrol and novel potent activators of Sirt1: effects on aging and age-related diseases. Nutr Rev. 2008. 66:591–596.
Article2. Kaeberlein M, McVey M, Guarente L. The SIR2/3/4 complex and SIR2 alone promote longevity in Saccharomyces cerevisiae by two different mechanisms. Genes Dev. 1999. 13:2570–2580.
Article3. Yamamoto H, Schoonjans K, Auwerx J. Sirtuin functions in health and disease. Mol Endocrinol. 2007. 21:1745–1755.
Article4. Jiang WJ. Sirtuins: novel targets for metabolic disease in drug development. Biochem Biophys Res Commun. 2008. 373:341–344.
Article5. Houtkooper RH, Canto C, Wanders RJ, Auwerx J. The secret life of NAD+: an old metabolite controlling new metabolic signaling pathways. Endocr Rev. 2009. 31:194–223.
Article6. Kong XX, Wang R, Liu XJ, Zhu LL, Shao D, Chang YS, et al. Function of Sirt1 in physiology. Biochemistry. 2009. 74:703–708.
Article7. Yu J, Auwerx J. The role of sirtuins in the control of metabolic homeostasis. Ann N Y Acad Sci. 2009. 1173:Suppl 1. E10–E19.
Article8. Longo VD, Kennedy BK. Sirtuins in aging and age-related disease. Cell. 2006. 126:257–268.
Article9. Lin SJ, Defossez PA, Guarente L. Requirement of NAD and SIR2 for life-span extension by calorie restriction in Saccharomyces cerevisiae. Science. 2000. 289:2126–2128.
Article10. Hallows WC, Lee S, Denu JM. Sirtuins deacetylate and activate mammalian acetyl-CoA synthetases. Proc Natl Acad Sci U S A. 2006. 103:10230–10235.
Article11. Rodgers JT, Puigserver P. Fasting-dependent glucose and lipid metabolic response through hepatic sirtuin 1. Proc Natl Acad Sci U S A. 2007. 104:12861–12866.
Article12. Li X, Zhang S, Blander G, Tse JG, Krieger M, Guarente L. Sirt1 deacetylates and positively regulates the nuclear receptor LXR. Mol Cell. 2007. 28:91–106.
Article13. Baur JA, Pearson KJ, Price NL, Jamieson HA, Lerin C, Kalra A, et al. Resveratrol improves health and survival of mice on a high-calorie diet. Nature. 2006. 444:337–342.
Article14. Lagouge M, Argmann C, Gerhart-Hines Z, Meziane H, Lerin C, Daussin F, et al. Resveratrol improves mitochondrial function and protects against metabolic disease by activating Sirt1 and PGC-1alpha. Cell. 2006. 127:1109–1122.
Article15. Imai S, Kiess W. Therapeutic potential of Sirt1 and NAMPT-mediated NAD biosynthesis in type 2 diabetes. Front Biosci. 2009. 14:2983–2995.
Article16. Feige JN, Lagouge M, Canto C, Strehle A, Houten SM, Milne JC, et al. Specific Sirt1 activation mimics low energy levels and protects against diet-induced metabolic disorders by enhancing fat oxidation. Cell Metab. 2008. 8:347–358.
Article17. Moynihan KA, Grimm AA, Plueger MM, Bernal-Mizrachi E, Ford E, Cras-Meneur C, et al. Increased dosage of mammalian Sir2 in pancreatic beta cells enhances glucose-stimulated insulin secretion in mice. Cell Metab. 2005. 2:105–117.
Article18. Picard F, Kurtev M, Chung N, Topark-Ngarm A, Senawong T, Machado De Oliveira R, et al. Sirt1 promotes fat mobilization in white adipocytes by repressing PPAR-gamma. Nature. 2004. 429:771–776.
Article19. Zabolotny JM, Kim YB. Silencing insulin resistance through Sirt1. Cell Metab. 2007. 6:247–249.
Article20. Sun C, Zhang F, Ge X, Yan T, Chen X, Shi X, et al. Sirt1 improves insulin sensitivity under insulin-resistant conditions by repressing PTP1B. Cell Metab. 2007. 6:307–319.
Article21. Bordone L, Cohen D, Robinson A, Motta MC, van Veen E, Czopik A, et al. Sirt1 transgenic mice show phenotypes resembling calorie restriction. Aging Cell. 2007. 6:759–767.
Article22. Ying W. NAD+ and NADH in cellular functions and cell death. Front Biosci. 2006. 11:3129–3148.
Article23. van der Veer E, Ho C, O'Neil C, Barbosa N, Scott R, Cregan SP, et al. Extension of human cell lifespan by nicotinamide phosphoribosyltransferase. J Biol Chem. 2007. 282:10841–10845.
Article24. Kim JE, Chen J, Lou Z. DBC1 is a negative regulator of Sirt1. Nature. 2008. 451:583–586.
Article25. Yang Y, Fu W, Chen J, Olashaw N, Zhang X, Nicosia SV, et al. Sirt1 sumoylation regulates its deacetylase activity and cellular response to genotoxic stress. Nat Cell Biol. 2007. 9:1253–1262.
Article26. Kim EJ, Kho JH, Kang MR, Um SJ. Active regulator of Sirt1 cooperates with Sirt1 and facilitates suppression of p53 activity. Mol Cell. 2007. 28:277–290.
Article27. Zhou G, Sebhat IK, Zhang BB. AMPK activators--potential therapeutics for metabolic and other diseases. Acta Physiol. 2009. 196:175–190.28. Hegarty BD, Turner N, Cooney GJ, Kraegen EW. Insulin resistance and fuel homeostasis: the role of AMP-activated protein kinase. Acta Physiol (Oxf). 2009. 196:129–145.
Article29. Fulco M, Sartorelli V. Comparing and contrasting the roles of AMPK and Sirt1 in metabolic tissues. Cell Cycle. 2008. 7:3669–3679.
Article30. Apfeld J, O'Connor G, McDonagh T, DiStefano PS, Curtis R. The AMP-activated protein kinase AAK-2 links energy levels and insulin-like signals to lifespan in C. elegans. Genes Dev. 2004. 18:3004–3009.
Article31. Jager S, Handschin C, St-Pierre J, Spiegelman BM. AMP-activated protein kinase (AMPK) action in skeletal muscle via direct phosphorylation of PGC-1alpha. Proc Natl Acad Sci USA. 2007. 104:12017–12022.32. Canto C, Gerhart-Hines Z, Feige JN, Lagouge M, Noriega L, Milne JC, et al. AMPK regulates energy expenditure by modulating NAD+ metabolism and Sirt1 activity. Nature. 2009. 458:1056–1060.
Article33. Rivera L, Moron R, Zarzuelo A, Galisteo M. Long-term resveratrol administration reduces metabolic disturbances and lowers blood pressure in obese Zucker rats. Biochem Pharmacol. 2009. 77:1053–1063.
Article34. Barger JL, Kayo T, Vann JM, Arias EB, Wang J, Hacker TA, et al. A low dose of dietary resveratrol partially mimics caloric restriction and retards aging parameters in mice. PLoS One. 2008. 3:e2264.
Article35. Szkudelska K, Szkudelski T. Resveratrol, obesity and diabetes. Eur J Pharmacol. 2010. 635:1–8.
Article36. Milne JC, Lambert PD, Schenk S, Carney DP, Smith JJ, Gagne DJ, et al. Small molecule activators of Sirt1 as therapeutics for the treatment of type 2 diabetes. Nature. 2007. 450:712–716.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- SIRT1 in Type 2 Diabetes: Mechanisms and Therapeutic Potential
- Potential Role of Sirtuin as a Therapeutic Target for Neurodegenerative Diseases
- As a Modulator, Multitasking Roles of SIRT1 in Respiratory Diseases
- Aging-Related Correlation between Serum Sirtuin 1 Activities and Basal Metabolic Rate in Women, but not in Men
- Calorie Restriction and Obesity under the Regulation of SIRT1