Yonsei Med J.
2013 May;54(3):791-796. 10.3349/ymj.2013.54.3.791.
Primary Central Nervous System ALK Positive Anaplastic Large Cell Lymphoma with Predominantly Leptomeningeal Involvement in an Adult
- Affiliations
-
- 1Department of Neurosurgery, Samsung Medical Center, Sungkyunkwan University School of Medicine, Seoul, Korea.
- 2Department of Pathology, Samsung Medical Center, Sungkyunkwan University School of Medicine, Seoul, Korea. yhko310@skku.edu
- 3Department of Internal Medicine, Samsung Medical Center, Sungkyunkwan University School of Medicine, Seoul, Korea.
- 4Department of Neurosurgery, Konkuk University Chungju Hospital, Chungju, Korea.
- 5Department of Pathology, Ewha Womans University School of Medicine, Seoul, Korea.
- KMID: 1727900
- DOI: http://doi.org/10.3349/ymj.2013.54.3.791
Abstract
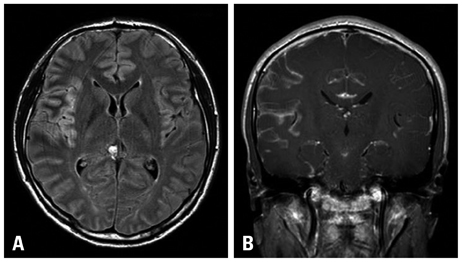
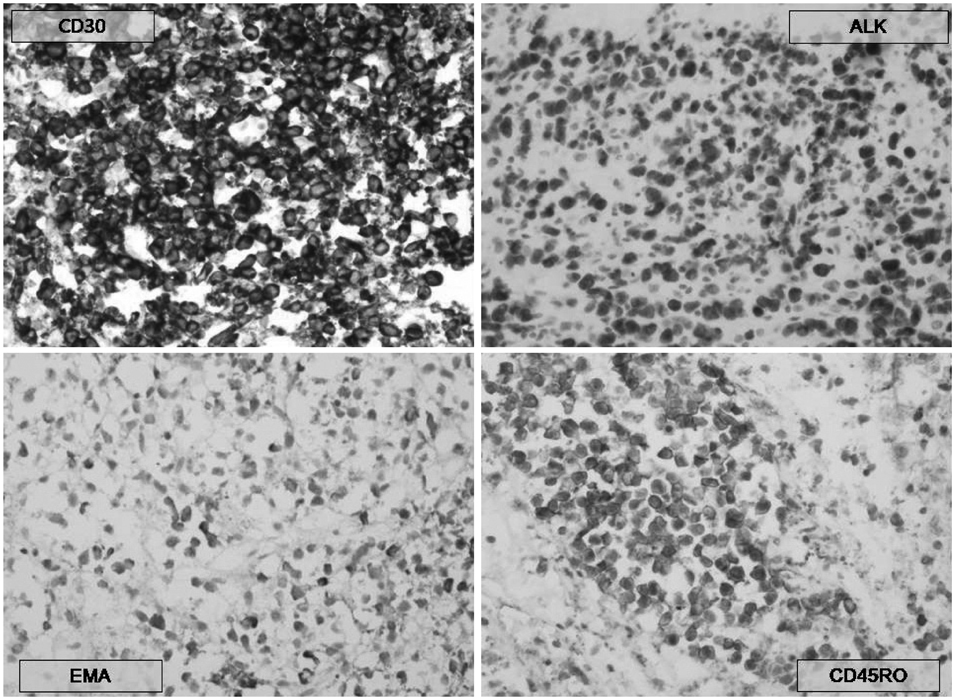
- A 31-year-old Korean male presented with altered consciousness and severe headache. Brain MRI delineated focal leptomeningeal enhancement without any intracerebral lesions. Diagnosis was made based on a brain biopsy showing anaplastic large cell lymphoma (ALCL), immunohistochemical stains revealing positivity for anaplastic lymphoma kinase (ALK) and an absence of involvement in any other organs; specifically, the primary central nervous system ALK+ALCL. Complete remission was achieved following 5 cycles of systemic chemotherapy with a high dose of Methotrexate and a simultaneous 7 cycles of intrathecal triple chemotherapy. Diagnosis of primary leptomeningeal ALK+ALCL is challenging given its rarity and non-specific symptoms along with non-pathognomonic radiologic findings. We present the first case of primary leptomeningeal ALK-positive ALCL where the clinical course, pathologic characteristics and treatment modality are described as well as a review of literature.
MeSH Terms
-
Adult
Antineoplastic Agents/therapeutic use
Biopsy
Brain/metabolism/pathology
Diagnosis, Differential
Humans
Immunohistochemistry
Lymphoma, Large-Cell, Anaplastic/*diagnosis/drug therapy/pathology
Male
Meningeal Neoplasms/*diagnosis/drug therapy/pathology
Receptor Protein-Tyrosine Kinases/*metabolism
Antineoplastic Agents
Receptor Protein-Tyrosine Kinases
Figure
Reference
-
1. Stein H, Mason DY, Gerdes J, O'Connor N, Wainscoat J, Pallesen G, et al. The expression of the Hodgkin's disease associated antigen Ki-1 in reactive and neoplastic lymphoid tissue: evidence that Reed-Sternberg cells and histiocytic malignancies are derived from activated lymphoid cells. Blood. 1985. 66:848–858.2. Jaffe ES, Harris NL, Stein H, Wardiman JW. Tumors of haematopoietic and lymphoid tissues. 2001. Lyon: IARC Press.3. Campo E, Swerdlow SH, Harris NL, Pileri S, Stein H, Jaffe ES. The 2008 WHO classification of lymphoid neoplasms and beyond: evolving concepts and practical applications. Blood. 2011. 117:5019–5032.
Article4. Swerdlow SH, Campo E, Harris NL, Jaffe ES, Pileri SA, Stein H. WHO Classification of Tumours of Haematopoietic and Lymphoid Tissues. 2008. 4th ed. Lyon, France: IARC Press.5. Karikari IO, Thomas KK, Lagoo A, Cummings TJ, George TM. Primary cerebral ALK-1-positive anaplastic large cell lymphoma in a child. Case report and literature review. Pediatr Neurosurg. 2007. 43:516–521.
Article6. Penny RJ, Blaustein JC, Longtine JA, Pinkus GS. Ki-1-positive large cell lymphomas, a heterogenous group of neoplasms. Morphologic, immunophenotypic, genotypic, and clinical features of 24 cases. Cancer. 1991. 68:362–373.
Article7. Falini B, Pileri S, Zinzani PL, Carbone A, Zagonel V, Wolf-Peeters C, et al. ALK+ lymphoma: clinico-pathological findings and outcome. Blood. 1999. 93:2697–2706.8. Abdulkader I, Cameselle-Teijeiro J, Fraga M, Rodriguez-Núnez A, Allut AG, Forteza J. Primary anaplastic large cell lymphoma of the central nervous system. Hum Pathol. 1999. 30:978–981.
Article9. George DH, Scheithauer BW, Aker FV, Kurtin PJ, Burger PC, Cameselle-Teijeiro J, et al. Primary anaplastic large cell lymphoma of the central nervous system: prognostic effect of ALK-1 expression. Am J Surg Pathol. 2003. 27:487–493.10. Ponzoni M, Terreni MR, Ciceri F, Ferreri AJ, Gerevini S, Anzalone N, et al. Primary brain CD30+ ALK1+ anaplastic large cell lymphoma ('ALKoma'): the first case with a combination of 'not common' variants. Ann Oncol. 2002. 13:1827–1832.
Article11. Merlin E, Chabrier S, Verkarre V, Cramer E, Delabesse E, Stéphan JL. Primary leptomeningeal ALK+ lymphoma in a 13-year-old child. J Pediatr Hematol Oncol. 2008. 30:963–967.
Article12. Ozkaynak MF. Favorable outcome of primary CNS anaplastic large cell lymphoma in an immunocompetent patient. J Pediatr Hematol Oncol. 2009. 31:128–130.
Article13. Shiota M, Nakamura S, Ichinohasama R, Abe M, Akagi T, Takeshita M, et al. Anaplastic large cell lymphomas expressing the novel chimeric protein p80NPM/ALK: a distinct clinicopathologic entity. Blood. 1995. 86:1954–1960.
Article14. Villano JL, Koshy M, Shaikh H, Dolecek TA, McCarthy BJ. Age, gender, and racial differences in incidence and survival in primary CNS lymphoma. Br J Cancer. 2011. 105:1414–1418.
Article15. Buxton N, Punt J, Hewitt M. Primary Ki-1-positive T-cell lymphoma of the brain in a child. Pediatr Neurosurg. 1998. 29:250–252.
Article16. Goldbrunner R, Warmuth-Metz M, Tonn JC, Vince GH, Roosen K. Primary Ki-1-positive T-cell lymphoma of the brain--an aggressive subtype of lymphoma: case report and review of the literature. Surg Neurol. 1996. 46:37–41.
Article17. Paulus W, Ott MM, Strik H, Keil V, Müller-Hermelink HK. Large cell anaplastic (KI-1) brain lymphoma of T-cell genotype. Hum Pathol. 1994. 25:1253–1256.
Article18. Lachance DH, O'Neill BP, Macdonald DR, Jaeckle KA, Witzig TE, Li CY, et al. Primary leptomeningeal lymphoma: report of 9 cases, diagnosis with immunocytochemical analysis, and review of the literature. Neurology. 1991. 41:95–100.
Article19. Taga T, Sakaue Y, Anzai Y, Takeuchi Y, Ohta S. Pediatric primary leptomeningeal lymphoma treated without cranial radiotherapy. Pediatr Blood Cancer. 2007. 48:477–478.
Article20. Felice MS, Zubizarreta PA, Rossi JG, Rose A, Alfaro EM, Sackmann-Muriel F. Diagnosis and successful treatment of childhood primary leptomeningeal lymphoma. Med Pediatr Oncol. 2000. 34:361–363.
Article21. Shah AC, Kelly DR, Nabors LB, Oakes WJ, Hilliard LM, Reddy AT. Treatment of primary CNS lymphoma with high-dose methotrexate in immunocompetent pediatric patients. Pediatr Blood Cancer. 2010. 55:1227–1230.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- A Case of Multiple Cranial Neuropathies Caused by Anaplastic Lymphoma Kinase-Negative Anaplastic Large Cell Lymphoma
- CD30-Positive Anaplastic Lymphoma Kinase-Negative Systemic Anaplastic Large-Cell Lymphoma in a 9-Year-Old Boy
- A Case of ALK-Negative Systemic Anaplastic Large Cell Lymphoma
- A Case of CD30 (+)/ALK (-) Primary Systemic AnaplasticLarge Cell Lymphoma with Atypical Clinical Features
- A Case of CD30 Positive ALK-Negative Systemic Anaplastic Large Cell Lymphoma Involving Bone Marrow