Yonsei Med J.
2013 May;54(3):707-714. 10.3349/ymj.2013.54.3.707.
Kinetics of IFN-Gamma and TNF-Alpha Gene Expression and Their Relationship with Disease Progression after Infection with Mycobacterium Tuberculosis in Guinea Pigs
- Affiliations
-
- 1Department of Microbiology, Yonsei University College of Medicine, Seoul, Korea. raycho@yuhs.ac
- 2Institute of Immunology and Immunological Diseases, Yonsei University College of Medicine, Seoul, Korea.
- 3Animal, Plant and Fisheries Quarantine and Inspection Agency, Anyang, Korea.
- 4Basic Science Institute for Cell Damage Control, Sogang University, Seoul, Korea.
- 5Division of Immunopathology and Cellular Immunology, International Tuberculosis Research Center, Changwon, Korea.
- KMID: 1727887
- DOI: http://doi.org/10.3349/ymj.2013.54.3.707
Abstract
- PURPOSE
Guinea pig is one of the most suitable animal models for Mycobacterium tuberculosis (M. tb) infection since it shows similarities to pulmonary infection in humans. Although guinea pig shows hematogenous spread of M. tb infection into the whole body, immunological studies have mainly focused on granulomatous tissues in lungs and spleens. In order to investigate the time-course of disease pathogenesis and immunological profiles in each infected organ, we performed the following approaches with guinea pigs experimentally infected with M. tb over a 22-week post-infection period.
MATERIALS AND METHODS
We examined body weight changes, M. tb growth curve, cytokine gene expression (IFN-gamma and TNF-alpha), and histopathology in liver, spleen, lungs and lymph nodes of infected guinea pigs.
RESULTS
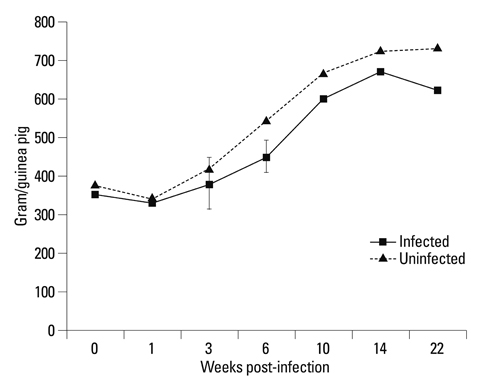
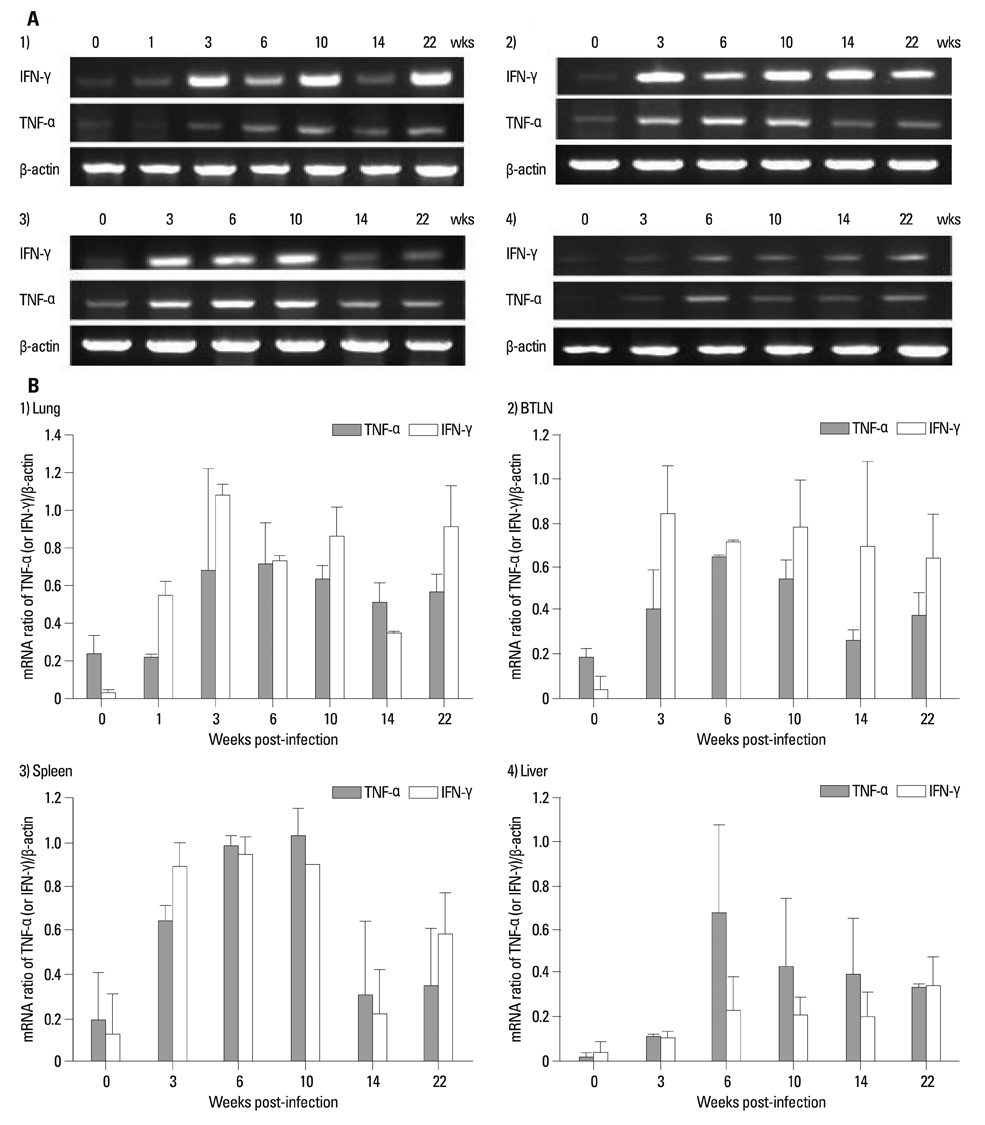
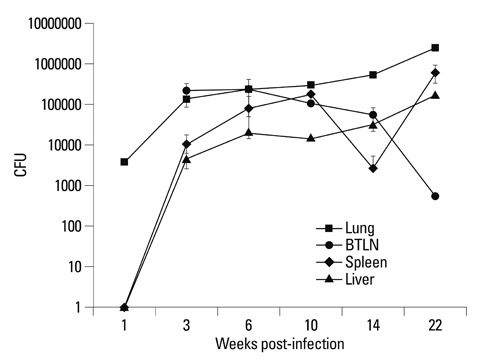
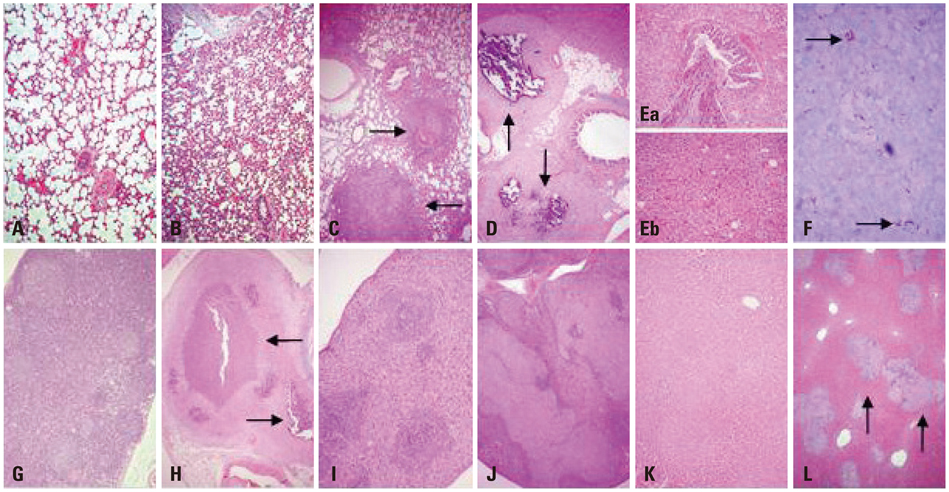
The body weights of infected guinea pigs did not increase as much as uninfected ones and the number of M. tb bacilli in their organs increased except bronchotracheal lymph node during the experimental period. The gene expression of IFN-gamma and TNF-alpha was induced between 3 and 6 weeks of infection; however, kinetic profiles of cytokine gene expression showed heterogeneity among organs over the study period. Histophathologically granulomatous lesions were developed in all four organs of infected guinea pigs.
CONCLUSION
Although IFN-gamma and TNF-alpha gene expression profiles showed heterogeneity, the granuloma formation was clearly observed in every organ regardless of whether the number of bacilli increased or decreased. However, this protective immunity was accompanied with severe tissue damage in all four organs, which may lead to the death of guinea pigs.
MeSH Terms
-
Animals
Body Weight
*Disease Progression
Female
Gene Expression
Gene Expression Regulation
Guinea Pigs
Interferon-gamma/genetics/*metabolism
Kinetics
Liver/metabolism/pathology
Lung/metabolism/pathology
Lymph Nodes/metabolism/pathology
Mycobacterium tuberculosis
Spleen/metabolism/pathology
Tuberculosis/*genetics/pathology
Tumor Necrosis Factor-alpha/genetics/*metabolism
Tumor Necrosis Factor-alpha
Interferon-gamma
Figure
Cited by 1 articles
-
Whole Blood Interferon-γ Release Assay Is Insufficient for the Diagnosis of Sputum Smear Negative Pulmonary Tuberculosis
HeeJin Park, Jung Ar Shin, Hyung Jung Kim, Chul Min Ahn, Yoon Soo Chang
Yonsei Med J. 2014;55(3):725-731. doi: 10.3349/ymj.2014.55.3.725.
Reference
-
1. Tuberculosis control and research strategies for the 1990s: memo-randum from a WHO meeting. Bull World Health Organ. 1992. 70:17–21.2. Corbett EL, Watt CJ, Walker N, Maher D, Williams BG, Raviglione MC, et al. The growing burden of tuberculosis: global trends and interactions with the HIV epidemic. Arch Intern Med. 2003. 163:1009–1021.3. WHO. Global tuberculosis report 2011. Available from: http://www.who.int/tb/publications/global_report/2011/en/index.html.4. Roche PW, Triccas JA, Winter N. BCG vaccination against tuberculosis: past disappointments and future hopes. Trends Microbiol. 1995. 3:397–401.
Article5. Karonga Prevention Trial Group. Randomised controlled trial of single BCG, repeated BCG, or combined BCG and killed Mycobacterium leprae vaccine for prevention of leprosy and tuberculosis in Malawi. Lancet. 1996. 348:17–24.6. McShane H. Tuberculosis vaccines: beyond bacille Calmette-Guerin. Philos Trans R Soc Lond B Biol Sci. 2011. 366:2782–2789.7. Rowland R, McShane H. Tuberculosis vaccines in clinical trials. Expert Rev Vaccines. 2011. 10:645–658.
Article8. Shah NS, Wright A, Bai GH, Barrera L, Boulahbal F, Martín-Casabona N, et al. Worldwide emergence of extensively drug-resistant tuberculosis. Emerg Infect Dis. 2007. 13:380–387.
Article9. Chan ED, Iseman MD. Multidrug-resistant and extensively drug-resistant tuberculosis: a review. Curr Opin Infect Dis. 2008. 21:587–595.
Article10. Barry CE 3rd, Blanchard JS. The chemical biology of new drugs in the development for tuberculosis. Curr Opin Chem Biol. 2010. 14:456–466.
Article11. Shin SS, Keshavjee S, Gelmanova IY, Atwood S, Franke MF, Mishustin SP, et al. Development of extensively drug-resistant tuberculosis during multidrug-resistant tuberculosis treatment. Am J Respir Crit Care Med. 2010. 182:426–432.
Article12. Gandhi NR, Moll A, Sturm AW, Pawinski R, Govender T, Lalloo U, et al. Extensively drug-resistant tuberculosis as a cause of death in patients co-infected with tuberculosis and HIV in a rural area of South Africa. Lancet. 2006. 368:1575–1580.
Article13. Ma Z, Lienhardt C, McIlleron H, Nunn AJ, Wang X. Global tuberculosis drug development pipeline: the need and the reality. Lancet. 2010. 375:2100–2109.
Article14. Dannenberg AM Jr. Perspectives on clinical and preclinical testing of new tuberculosis vaccines. Clin Microbiol Rev. 2010. 23:781–794.
Article15. Cole ST, Riccardi G. New tuberculosis drugs on the horizon. Curr Opin Microbiol. 2011. 14:570–576.
Article16. Okada M, Kita Y. Tuberculosis vaccine development: the development of novel (preclinical) DNA vaccine. Hum Vaccin. 2010. 6:297–308.
Article17. Checkley AM, McShane H. Tuberculosis vaccines: progress and challenges. Trends Pharmacol Sci. 2011. 32:601–606.
Article18. Smith DW, Balasubramanian V, Wiegeshaus E. A guinea pig model of experimental airborne tuberculosis for evaluation of the response to chemotherapy: the effect on bacilli in the initial phase of treatment. Tubercle. 1991. 72:223–231.
Article19. Dannenberg AM Jr, Collins FM. Progressive pulmonary tuberculosis is not due to increasing numbers of viable bacilli in rabbits, mice and guinea pigs, but is due to a continuous host response to mycobacterial products. Tuberculosis (Edinb). 2001. 81:229–242.
Article20. McMurray DN, Allen SS, Jeevan A, Lasco T, Cho H, Skwor T, et al. Vaccine-induced cytokine responses in a guinea pig model of pulmonary tuberculosis. Tuberculosis (Edinb). 2005. 85:295–301.
Article21. Sugawara I, Udagawa T, Aoki T, Mizuno S. Establishment of a guinea pig model of latent tuberculosis with GFP-introduced Mycobacterium tuberculosis. Tohoku J Exp Med. 2009. 219:257–262.
Article22. Young D. Animal models of tuberculosis. Eur J Immunol. 2009. 39:2011–2014.
Article23. Ahmad Z, Fraig MM, Pinn ML, Tyagi S, Nuermberger EL, Grosset JH, et al. Effectiveness of tuberculosis chemotherapy correlates with resistance to Mycobacterium tuberculosis infection in animal models. J Antimicrob Chemother. 2011. 66:1560–1566.
Article24. Grover A, Troudt J, Arnett K, Izzo L, Lucas M, Strain K, et al. Assessment of vaccine testing at three laboratories using the guinea pig model of tuberculosis. Tuberculosis (Edinb). 2012. 92:105–111.
Article25. Salgame P. Host innate and Th1 responses and the bacterial factors that control Mycobacterium tuberculosis infection. Curr Opin Immunol. 2005. 17:374–380.
Article26. Cooper AM. T cells in mycobacterial infection and disease. Curr Opin Immunol. 2009. 21:378–384.
Article27. Jeon BY, Eoh H, Ha SJ, Bang H, Kim SC, Sung YC, et al. Co-immunization of plasmid DNA encoding IL-12 and IL-18 with Bacillus Calmette-Guérin vaccine against progressive tuberculosis. Yonsei Med J. 2011. 52:1008–1015.
Article28. North RJ, Jung YJ. Immunity to tuberculosis. Annu Rev Immunol. 2004. 22:599–623.29. Gupta A, Kaul A, Tsolaki AG, Kishore U, Bhakta S. Mycobacterium tuberculosis: immune evasion, latency and reactivation. Immunobiology. 2012. 217:363–374.
Article30. Brighenti S, Andersson J. Local immune responses in human tuberculosis: learning from the site of infection. J Infect Dis. 2012. 205:Suppl 2. S316–S324.
Article31. Orme IM, Cooper AM. Cytokine/chemokine cascades in immunity to tuberculosis. Immunol Today. 1999. 20:307–312.
Article32. Rahman S, Gudetta B, Fink J, Granath A, Ashenafi S, Aseffa A, et al. Compartmentalization of immune responses in human tuberculosis: few CD8+ effector T cells but elevated levels of FoxP3+ regulatory t cells in the granulomatous lesions. Am J Pathol. 2009. 174:2211–2224.33. Kawahara M, Nakasone T, Honda M. Dynamics of gamma interferon, interleukin-12 (IL-12), IL-10, and transforming growth factor beta mRNA expression in primary Mycobacterium bovis BCG infection in guinea pigs measured by a real-time fluorogenic reverse transcription-PCR assay. Infect Immun. 2002. 70:6614–6620.
Article34. Cho H, Lasco TM, Allen SS, Yoshimura T, McMurray DN. Recombinant guinea pig tumor necrosis factor alpha stimulates the expression of interleukin-12 and the inhibition of Mycobacterium tuberculosis growth in macrophages. Infect Immun. 2005. 73:1367–1376.
Article35. McMurray DN. Hematogenous reseeding of the lung in low-dose, aerosol-infected guinea pigs: unique features of the host-pathogen interface in secondary tubercles. Tuberculosis (Edinb). 2003. 83:131–134.
Article36. Cooper AM, Dalton DK, Stewart TA, Griffin JP, Russell DG, Orme IM. Disseminated tuberculosis in interferon gamma gene-disrupted mice. J Exp Med. 1993. 178:2243–2247.
Article37. Flynn JL, Chan J, Triebold KJ, Dalton DK, Stewart TA, Bloom BR. An essential role for interferon gamma in resistance to Mycobacterium tuberculosis infection. J Exp Med. 1993. 178:2249–2254.
Article38. Ly LH, Russell MI, McMurray DN. Cytokine profiles in primary and secondary pulmonary granulomas of Guinea pigs with tuberculosis. Am J Respir Cell Mol Biol. 2008. 38:455–462.
Article39. Ly LH, Russell MI, McMurray DN. Microdissection of the cytokine milieu of pulmonary granulomas from tuberculous guinea pigs. Cell Microbiol. 2007. 9:1127–1136.
Article40. Chackerian AA, Alt JM, Perera TV, Dascher CC, Behar SM. Dissemination of Mycobacterium tuberculosis is influenced by host factors and precedes the initiation of T-cell immunity. Infect Immun. 2002. 70:4501–4509.
Article41. Fenhalls G, Stevens L, Bezuidenhout J, Amphlett GE, Duncan K, Bardin P, et al. Distribution of IFN-gamma, IL-4 and TNF-alpha protein and CD8 T cells producing IL-12p40 mRNA in human lung tuberculous granulomas. Immunology. 2002. 105:325–335.
Article42. Fuller CL, Flynn JL, Reinhart TA. In situ study of abundant expression of proinflammatory chemokines and cytokines in pulmonary granulomas that develop in cynomolgus macaques experimentally infected with Mycobacterium tuberculosis. Infect Immun. 2003. 71:7023–7034.
Article43. Aung H, Toossi Z, McKenna SM, Gogate P, Sierra J, Sada E, et al. Expression of transforming growth factor-beta but not tumor necrosis factor-alpha, interferon-gamma, and interleukin-4 in granulomatous lung lesions in tuberculosis. Tuber Lung Dis. 2000. 80:61–67.
Article44. Dai G, McMurray DN. Effects of modulating TGF-beta 1 on immune responses to mycobacterial infection in guinea pigs. Tuber Lung Dis. 1999. 79:207–214.
Article45. Fenhalls G, Wong A, Bezuidenhout J, van Helden P, Bardin P, Lukey PT. In situ production of gamma interferon, interleukin-4, and tumor necrosis factor alpha mRNA in human lung tuberculous granulomas. Infect Immun. 2000. 68:2827–2836.
Article46. Allen SS, Cassone L, Lasco TM, McMurray DN. Effect of neutralizing transforming growth factor beta1 on the immune response against Mycobacterium tuberculosis in guinea pigs. Infect Immun. 2004. 72:1358–1363.
Article47. Grover A, Taylor J, Troudt J, Keyser A, Arnett K, Izzo L, et al. Kinetics of the immune response profile in guinea pigs after vaccination with Mycobacterium bovis BCG and infection with Mycobacterium tuberculosis. Infect Immun. 2009. 77:4837–4846.
Article48. Jeevan A, Yoshimura T, Ly LH, Dirisala VR, McMurray DN. Cloning of guinea pig IL-4: reduced IL-4 mRNA after vaccination or Mycobacterium tuberculosis infection. Tuberculosis (Edinb). 2011. 91:47–56.
Article49. Dirisala VR, Jeevan A, Bix G, Yoshimura T, McMurray DN. Molecular cloning and expression of the IL-10 gene from guinea pigs. Gene. 2012. 498:120–127.
Article50. Iwakura Y, Nakae S, Saijo S, Ishigame H. The roles of IL-17A in inflammatory immune responses and host defense against pathogens. Immunol Rev. 2008. 226:57–79.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- The priming effect of IFN-gamma and numbers of IFN-gamma receptors in patients with chronic refractory tuberculosis
- Change of IFN-g and TNF-a Producing Capacity in the Course of Chemotherapy in Patients with Pulmonary Tuberculosis
- The Functional and Genetic Defects of IFN-gamma Receptor in the Patients with Tuberculosis
- The Comparison of Cellular Immune Reactions in Mycobacterium Tuberculosis-Inoculated Guinea Pigs and Rabbits
- IFN-gammamRNA Expression in Tuberculous Pleural Lymphocytes After in vitro Stimulation with M. tuberculosis Antigens