J Vet Sci.
2009 Dec;10(4):361-363. 10.4142/jvs.2009.10.4.361.
Pathological features of bone marrow transplantation-related toxicity in a mouse
- Affiliations
-
- 1Institute of Veterinary Science, Chungnam National University, Daejeon 305-764, Korea. hyson@cnu.ac.kr
- 2Korea Institute of Toxicology, Daejeon 305-343, Korea.
- KMID: 1726912
- DOI: http://doi.org/10.4142/jvs.2009.10.4.361
Abstract
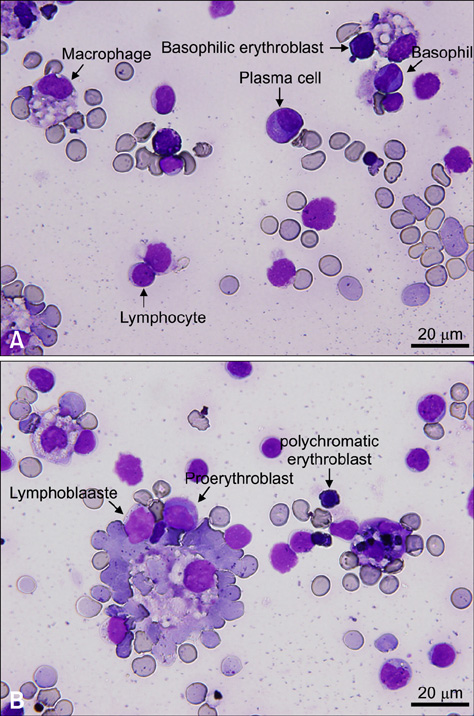
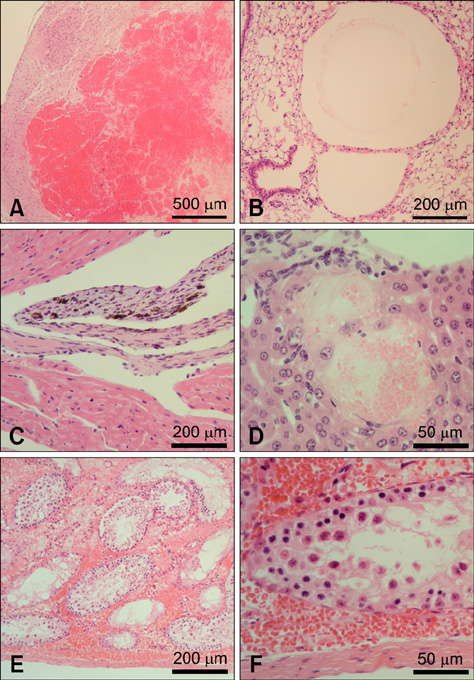
- In this case report, we present a mock-transduced bone marrow (BM) transplantation in a mouse, which was found moribund and autopsied to evaluate pathogenesis. Macroscopically, red discoloration of systemic organs was observed. Hematological values revealed a decrease in white blood cells, red blood cells, hematocrit, hemoglobin, and platelets, but an increase in reticulocytes. In BM cytology, hematopoietic cell lines were severely depleted. Histopathologically, hemorrhage in the cerebellar parenchyma, hemosiderin deposition and hemorrhage in the heart, necrosis and telangiectasia in liver, pulmonary parenchymal cysts, spermatogenic germ cells necrosis, atrophy and hemorrhage in testis, oligospermia and hemorrhage in the epididymis, and atrophy of BM, thymus and spleen were observed. In conclusion, autoimmune-like complications such as hematological value change, BM dysplasia and systemic hemorrhage appear to be the lethal cause of the mouse transplanted with mock-transduced BM.
MeSH Terms
Figure
Reference
-
1. Armitage JO. Bone marrow transplantation. N Engl J Med. 1994. 330:827–838.
Article2. Bleggi-Torres LF, de Medeiros BC, Werner B, Neto JZ, Loddo G, Pasquini R, de Medeiros CR. Neuropathological findings after bone marrow transplantation: an autopsy study of 180 cases. Bone Marrow Transplant. 2000. 25:301–307.
Article3. Bleggi-Torres LF, Werner B, Gasparetto EL, de Medeiros BC, Pasquini R, de Medeiros CR. Intracranial hemorrhage following bone marrow transplantation: an autopsy study of 58 patients. Bone Marrow Transplant. 2002. 29:29–32.
Article4. Gilman AL, Kooy NW, Atkins DL, Ballas Z, Rumelhart S, Holida M, Lee N, Goldman F. Complete heart block in association with graft-versus-host disease. Bone Marrow Transplant. 1998. 21:85–88.
Article5. Graus F, Saiz A, Sierra J, Arbaiza D, Rovira M, Carreras E, Tolosa E, Rozman C. Neurologic complications of autologous and allogeneic bone marrow transplantation in patients with leukemia: a comparative study. Neurology. 1996. 46:1004–1009.
Article6. Hakim F, Fowler DH, Shearer GM, Gress RE. Animal models of acute and chronic graft-versus-host disease. Curr Protoc Immunol. 2001. Chapter 4, Unit 4.3.
Article7. Haviernik P, Zhang Y, Bunting KD. Retroviral transduction of murine hematopoietic stem cells. Methods Mol Biol. 2008. 430:229–241.
Article8. Lapp WS, Ghayur T, Mendes M, Seddik M, Seemayer TA. The functional and histological basis for graft-versus-host-induced immunosuppression. Immunol Rev. 1985. 88:107–133.
Article9. Levy S, Nagler A, Okon S, Marmary Y. Parotid salivary gland dysfunction in chronic graft-versus-host disease (cGVHD): a longitudinal study in a mouse model. Bone Marrow Transplant. 2000. 25:1073–1078.
Article10. Liao P, Toro A, Min W, Lee S, Roifman CM, Grunebaum E. Lentivirus gene therapy for purine nucleoside phosphorylase deficiency. J Gene Med. 2008. 10:1282–1293.
Article11. Mehta J, Powles R, Singhal S, Horton C, Middleton G, Eisen T, Meller S, Pinkerton CR, Treleaven J. Early identification of patients at risk of death due to infections, hemorrhage, or graft failure after allogeneic bone marrow transplantation on the basis of the leukocyte counts. Bone Marrow Transplant. 1997. 19:349–355.
Article12. Platzbecker U, Klingel K, Thiede C, Freiberg-Richter J, Schuh D, Ehninger G, Kandolf R, Bornh-user M. Acute heart failure after allogeneic blood stem cell transplantation due to massive myocardial infiltration by cytotoxic T cells of donor origin. Bone Marrow Transplant. 2001. 27:107–109.
Article13. Räty JK, Lesch HP, Wirth T, Ylä-Herttuala S. Improving safety of gene therapy. Curr Drug Saf. 2008. 3:46–53.
Article14. Soubani AO, Miller KB, Hassoun PM. Pulmonary complications of bone marrow transplantation. Chest. 1996. 109:1066–1077.
Article15. Sprangers B, Van Wijmeersch B, Luyckx A, Sagaert X, De Somer L, Rutgeerts O, Lenaerts C, Landuyt W, Boeckx N, Dubois B, De Wolf-Peeters C, Waer M, Billiau AD. Allogeneic bone marrow transplantation and donor lymphocyte infusion in a mouse model of irradiation-induced myelodysplastic/myeloproliferation syndrome (MD/MPS): evidence for a graft-versus-MD/MPS effect. Leukemia. 2009. 23:340–349.
Article16. Wagner AM, Beier K, Christen E, Holländer GA, Krenger W. Leydig cell injury as a consequence of an acute graft-versus-host reaction. Blood. 2005. 105:2988–2990.
Article