J Vet Sci.
2009 Dec;10(4):299-304. 10.4142/jvs.2009.10.4.299.
Infrared spectroscopy characterization of normal and lung cancer cells originated from epithelium
- Affiliations
-
- 1Laboratory of Pharmacology, College of Veterinary Medicine, Seoul National University, Seoul 151-742, Korea.
- 2Research Institute and Hospital, National Cancer Center, Goyang 410-769, Korea.
- 3Department of Chemistry, Soongsil University, Seoul 156-743, Korea. sjoo@ssu.ac.kr
- 4Department of Mechatronics, Gwangju Institute of Science and Technology, Gwangju 500-712, Korea.
- KMID: 1726901
- DOI: http://doi.org/10.4142/jvs.2009.10.4.299
Abstract
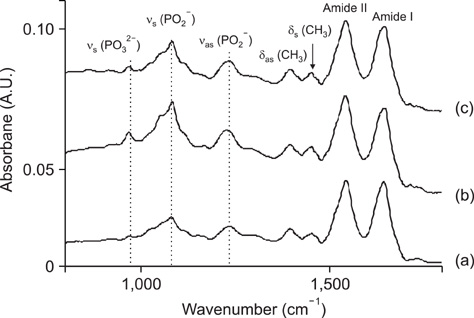
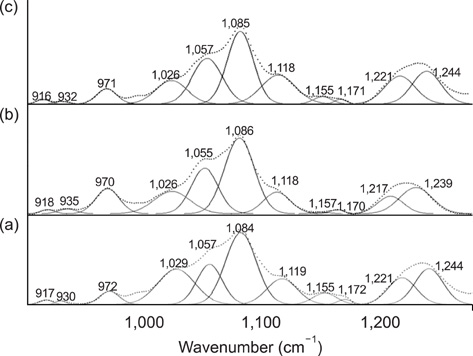
- The vibrational spectral differences of normal and lung cancer cells were studied for the development of effective cancer cell screening by means of attenuated total reflection infrared spectroscopy. The phosphate monoester symmetric stretching nus(PO3(2-)) band intensity at ~970 cm-1 and the phosphodiester symmetric stretching nus(PO2-) band intensity at ~1,085 cm-1 in nucleic acids and phospholipids appeared to be significantly strengthened in lung cancer cells with respect to the other vibrational bands compared to normal cells. This finding suggests that more extensive phosphorylation occur in cancer cells. These results demonstrate that lung cancer cells may be prescreened using infrared spectroscopy tools.
Keyword
MeSH Terms
Figure
Reference
-
1. Bode AM, Dong Z. Post-translational modification of p53 in tumorigenesis. Nat Rev Cancer. 2004. 4:793–805.
Article2. Choi J, Choo J, Chung H, Gweon DG, Park J, Kim HJ, Park S, Oh CH. Direct observation of spectral differences between normal and basal cell carcinoma (BCC) tissues using confocal Raman microscopy. Biopolymers. 2005. 77:264–272.
Article3. Cohenford MA, Rigas B. Cytologically normal cells from neoplastic cervical samples display extensive structural abnormalities on IR spectroscopy: Implications for tumor biology. Proc Natl Acad Sci USA. 1998. 95:15327–15332.
Article4. Dukor RK . Chalmers JM, Griffiths PR, editors. Vibrational spectroscopy in the detection of cancer. Handbook of Vibrational Spectroscopy. 2002. Vol. 5. New York: Wiley;3335–3361.5. Gazi E, Dwyer J, Lockyer NP, Gardner P, Shanks JH, Roulson J, Hart CA, Clarke NW, Brown MD. Biomolecular profiling of metastatic prostate cancer cells in bone marrow tissue using FTIR microspectroscopy: a pilot study. Anal Bioanal Chem. 2007. 387:1621–1631.
Article6. Hammiche A, German MJ, Hewitt R, Pollock HM, Martin FL. Monitoring cell cycle distributions in MCF-7 cells using near-field photothermal microspectroscopy. Biophys J. 2005. 88:3699–3706.
Article7. Hirsch FR, Franklin WA, Gazdar AF, Bunn PA Jr. Early detection of lung cancer: clinical perspectives of recent advances in biology and radiology. Clin Cancer Res. 2001. 7:5–22.8. Hunter T, Karin M. The regulation of transcription by phosphorylation. Cell. 1992. 70:375–387.
Article9. Ko LJ, Prives C. p53: puzzle and paradigm. Genes Dev. 1996. 10:1054–1072.
Article10. Kohlenberg E, Payette JR, Sowa MG, Levasseur MA, Riley CB, Leonardi L. Determining intestinal viability by near infrared spectroscopy: A veterinary application. Vib Spectrosc. 2005. 38:223–228.
Article11. Krishna CM, Kegelaer G, Adt I, Rubin S, Kartha VB, Manfait M, Sockalingum GD. Combined Fourier transform infrared and Raman spectroscopic approach for identification of multidrug resistance phenotype in cancer cell lines. Biopolymers. 2006. 82:462–470.
Article12. Lim JK, Lee Y, Lee K, Gong M, Joo SW. Reversible thermochromic change of molecular architecture for A diacetylene derivative 10,12-pentacosadiynoic acid thin films on Ag surfaces. Chem Lett. 2007. 36:1226–1227.
Article13. Liu C, Zhang Y, Yan X, Zhang X, Li C, Yang W, Shi D. Infrared absorption of human breast tissues in vitro. J Luminescence. 2006. 119-120:132–136.14. Maziak DE, Do MT, Shamji FM, Sundaresan SR, Perkins DG, Wong PTT. Fourier-transform infrared spectroscopic study of characteristic molecular structure in cancer cells of esophagus: An exploratory study. Cancer Detect Prev. 2007. 31:244–253.
Article15. Meurens M, Wallon J, Tong J, Noël H, Haot J. Breast cancer detection by Fourier transform infrared spectrometry. Vib Spectrosc. 1996. 10:341–346.
Article16. Mordechai S, Sahu RK, Hammody Z, Mark S, Kantarovich K, Guterman H, Podshyvalov A, Goldstein J, Argov S. Possible common biomarkers from FTIR microspectroscopy of cervical cancer and melanoma. J Microsc. 2004. 215:86–91.
Article17. Romeo M, Diem M. Correction of dispersive line shape artifact observed in diffuse reflection infrared spectroscopy and absorption/reflection (transflection) infrared microspectroscopy. Vib Spectrosc. 2005. 38:129–132.
Article18. Ruddon RW. Cancer Biology. 2007. Oxford: Oxford University Press;3–16.19. Sindhuphak R, Issaravanich S, Udomprasertgul V, Srisookho P, Warakamin S, Sindhuphak S, Boonbundarlchai R, Dusitsin N. A new approach for the detection of cervical cancer in Thai women. Gynecol Oncol. 2003. 90:10–14.
Article20. Sulé-Suso J, Skingsley D, Sockalingum GD, Kohler A, Kegelaer G, Manfait M, El Haj AJ. FT-IR microspectroscopy as a tool to assess lung cancer cells response to chemotherapy. Vib Spectrosc. 2005. 38:179–184.
Article21. Torrance CJ, Agrawal V, Vogelstein B, Kinzler KW. Use of isogenic human cancers cells for high-throughput screening and drug discovery. Nat Biotechnol. 2001. 19:940–945.
Article22. Walsh MJ, German MJ, Singh M, Pollock HM, Hammiche A, Kyrgiou M, Stringfellow HF, Paraskevaidis E, Martin-Hirsch PL, Martin FL. IR microspectroscopy: potential applications in cervical cancer screening. Cancer Lett. 2007. 246:1–11.
Article23. Wang HP, Wang HC, Huang YJ. Microscopic FTIR studies of lung cancer cells in pleural fluid. Sci Total Environ. 1997. 204:283–287.
Article24. Wong PTT, Wong RK, Caputo TA, Godwin TA, Rigas B. Infrared spectroscopy of exfoliated human cervical cells: evidence of extensive structural changes during carcinogenesis. Proc Natl Acad Sci USA. 1991. 88:10988–10992.
Article25. Zhang W, McClain C, Gau JP, Guo XYD, Deisseroth AB. Hyperphosphorylation of p53 induced by okadaic acid attenuates its transcriptional activation function. Cancer Res. 1994. 54:4448–4453.26. Zenobi MC, Luengo CV, Avena MJ, Rueda EH. An ATR-FTIR study of different phosphonic acids in aqueous solution. Spectrochim Acta A Mol Biomol Spectrosc. 2008. 70:270–276.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Clinical Application of Near Infrared Spectroscopy
- Two-channel Near-infrared Spectroscopic Analysis of Association of Paranoia Symptoms with Prefrontal Activation
- Calculi in Hydrocele: Incidence and Results of Infrared Spectroscopy Analysis
- Surgical Treatment of an Innominate Artery Aneurysm Using Near-Infrared Spectroscopy for Cerebral Monitoring: A Case Report
- Immunohistochemical Staining of Insulin-like Growth Factor-1 in Human Lung Cancer cells of NSCLC and SCLC