Korean J Gastroenterol.
2011 Aug;58(2):74-81. 10.4166/kjg.2011.58.2.74.
Primary Antibiotic Resistance of Helicobacter pylori Strains and Eradication Rate according to Gastroduodenal Disease in Korea
- Affiliations
-
- 1Department of Internal Medicine and Liver Research Institute, Seoul National University College of Medicine, Seoul, Korea. nayoungkim49@empal.com
- 2Department of Internal medicine, Seoul National University Bundang Hospital, Seongnam, Korea.
- 3Department of Microbiology, Hanyang University School of Medicine, Seoul, Korea.
- 4Digestive Endoscopic Center, Seoul Song Do Colorectal Hospital, Seoul, Korea.
- KMID: 1718413
- DOI: http://doi.org/10.4166/kjg.2011.58.2.74
Abstract
- BACKGROUND/AIMS
This study was performed to evaluate whether the prevalence rates of primary antibiotic resistance in Helicobacter pylori (H. pylori) isolates and the eradication rate of H. pylori could be different between cancer and non-cancer patients.
METHODS
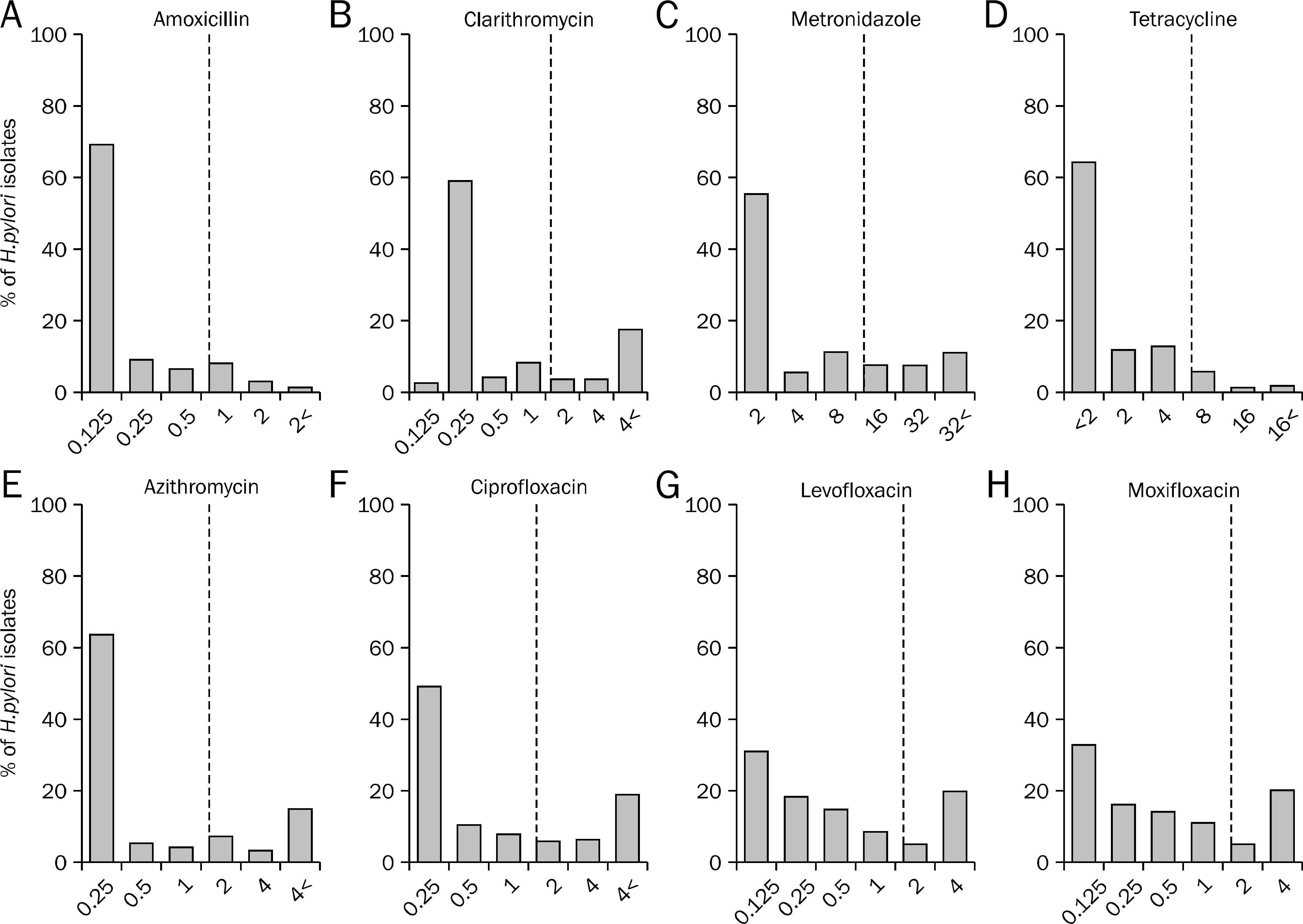
H. pylori were isolated from gastric mucosal biopsy specimens obtained from 269 Koreans, who did not have any eradication therapy history and were diagnosed as one of the following diseases; chronic gastritis, benign gastric ulcer, duodenal ulcer or gastric cancer. The susceptibilities of the H. pylori isolates to amoxicillin, clarithromycin, metronidazole, tetracycline, azithromycin, ciprofloxacin, levofloxacin and moxifloxacin were examined with the agar dilution method. In addition, eradication rate of H. pylori was evaluated.
RESULTS
There was no significant difference in the primary antibiotic resistance to above eight antibiotics among chronic gastritis, peptic ulcer disease and gastric cancer. Furthermore there was no difference of antibiotic resistance between cancer and non-cancer patients, and there was no difference of eradication rate of H. pylori according to disease.
CONCLUSIONS
Primary antibiotic resistance and H. pylori eradication rate were not different between cancer and non-cancer patients.
Keyword
MeSH Terms
-
2-Pyridinylmethylsulfinylbenzimidazoles/therapeutic use
Adult
Aged
Amoxicillin/therapeutic use
Anti-Bacterial Agents/therapeutic use
Chronic Disease
Clarithromycin/therapeutic use
*Drug Resistance, Bacterial
Drug Therapy, Combination
Duodenal Ulcer/complications/microbiology
Female
Gastritis/complications/microbiology
Helicobacter Infections/drug therapy/*epidemiology/microbiology
Helicobacter pylori/*drug effects/isolation & purification
Humans
Male
Microbial Sensitivity Tests
Middle Aged
Omeprazole/therapeutic use
Peptic Ulcer/complications/microbiology
Proton Pump Inhibitors/therapeutic use
Republic of Korea
Stomach Neoplasms/complications/microbiology
Figure
Reference
-
References
1. NIH Consensus Conference. Helicobacter pylori in peptic ulcer disease. NIH Consensus Development Panel on Helicobacter pylori in Peptic Ulcer Disease. JAMA. 1994; 272:65–69.2. Lind T, Mégraud F, Unge P, et al. The MACH2 study: role of omeprazole in eradication of Helicobacter pylori with 1-week triple therapies. Gastroenterology. 1999; 116:248–253.3. Malfertheiner P, Mégraud F, O'Morain C, et al. Current concepts in the management of Helicobacter pylori infection–the Maastricht 2-2000 Consensus Report. Aliment Pharmacol Ther. 2002; 16:167–180.4. Mégraud F. H. pylori antibiotic resistance: prevalence, im-portance, and advances in testing. Gut. 2004; 53:1374–1384.5. Kobayashi I, Murakami K, Kato M, et al. Changing antimicrobial susceptibility epidemiology of Helicobacter pylori strains in Japan between 2002 and 2005. J Clin Microbiol. 2007; 45:4006–4010.6. Kim JM, Kim JS, Jung HC, Kim N, Kim YJ, Song IS. Distribution of antibiotic MICs for Helicobacter pylori strains over a 16-year period in patients from Seoul, South Korea. Antimicrob Agents Chemother. 2004; 48:4843–4847.7. Kim N, Kim JM, Kim CH, et al. Institutional difference of antibiotic resistance of Helicobacter pylori strains in Korea. J Clin Gastroenterol. 2006; 40:683–687.8. Graham DY, Shiotani A. New concepts of resistance in the treatment of Helicobacter pylori infections. Nat Clin Pract Gastroenterol Hepatol. 2008; 5:321–331.9. Korean H. pylori Study Group. Diagnosis and treatment of Helicobacter pylori infection in Korea. Korean J Gastroenterol. 1998; 32:275–289.10. Uemura N, Mukai T, Okamoto S, et al. Effect of Helicobacter pylori eradication on subsequent development of cancer after endoscopic resection of early gastric cancer. Cancer Epidemiol Biomarkers Prev. 1997; 6:639–642.11. Uemura N, Okamoto S, Yamamoto S, et al. Helicobacter pylori infection and the development of gastric cancer. N Engl J Med. 2001; 345:784–789.12. Matsukura N, Tajiri T, Kato S, et al. Helicobacter pylori eradication therapy for the remnant stomach after gastrectomy. Gastric Cancer. 2003; 6:100–107.13. Kim N, Kim JJ, Choe YH, Kim HS, Kim JI, Chung IS. Diagnosis and treatment guidelines for Helicobacter pylori infection in Korea. Korean J Gastroenterol. 2009; 54:269–278.14. Gisbert JP, Marcos S, Gisbert JL, Pajares JM. Helicobacter pylori eradication therapy is more effective in peptic ulcer than in non-ulcer dyspepsia. Eur J Gastroenterol Hepatol. 2001; 13:1303–1307.15. Chung SJ, Lee DH, Kim N, et al. Eradication rates of Helicobacter pylori infection with second-line treatment: non-ulcer dyspepsia compared to peptic ulcer disease. Hepatogastroenterology. 2007; 54:1293–1296.16. Kim MN, Kim N, Lee SH, et al. The effects of probiotics on PPI-tri-ple therapy for Helicobacter pylori eradication. Helicobacter. 2008; 13:261–268.17. Jang S, Jones KR, Olsen CH, et al. Epidemiological link between gastric disease and polymorphisms in VacA and CagA. J Clin Microbiol. 2010; 48:559–567.
Article18. Kostamo P, Veijola L, Oksanen A, Sarna S, Rautelin H. Recent trends in primary antimicrobial resistance of Helicobacter pylori in Finland. Int J Antimicrob Agents. 2011; 37:22–25.19. Kim JY, Kim NY, Kim SJ, et al. Regional difference of antibiotic resistance of Helicobacter pylori strains in Korea. Korean J Gastroenterol. 2011; 57:221–229.20. Hwang TJ, Kim N, Kim HB, et al. Change in antibiotic resistance of Helicobacter pylori strains and the effect of A2143G point mu-tation of 23S rRNA on the eradication of H. pylori in a single center of Korea. J Clin Gastroenterol. 2010; 44:536–543.21. Gerrits MM, Schuijffel D, van Zwet AA, Kuipers EJ, Vanden-broucke-Grauls CM, Kusters JG. Alterations in penicillin-binding protein 1A confer resistance to beta-lactam antibiotics in Helicobacter pylori. Antimicrob Agents Chemother. 2002; 46:2229–2233.22. Kwon DH, Dore MP, Kim JJ, et al. High-level beta-lactam resistance associated with acquired multidrug resistance in Helicobacter pylori. Antimicrob Agents Chemother. 2003; 47:2169–2178.23. Ivashkin VT, Lapina TL, Bondarenko OY, et al. Azithromycin in a triple therapy for H. pylori eradication in active duodenal ulcer. World J Gastroenterol. 2002; 8:879–882.24. Gisbert JP, Morena F. Systematic review and meta-analysis: levo-floxacin-based rescue regimens after Helicobacter pylori treatment failure. Aliment Pharmacol Ther. 2006; 23:35–44.25. Li Y, Huang X, Yao L, Shi R, Zhang G. Advantages of Moxifloxacin and Levofloxacin-based triple therapy for second-line treatments of persistent Helicobacter pylori infection: a meta analysis. Wien Klin Wochenschr. 2010; 122:413–422.26. Yoon H, Kim N, Lee BH, et al. Moxifloxacin-containing triple therapy as second-line treatment for Helicobacter pylori infection: effect of treatment duration and antibiotic resistance on the eradication rate. Helicobacter. 2009; 14:77–85.27. Kim JJ, Kim JG, Kwon DH. Mixed-infection of antibiotic suscep-tible and resistant Helicobacter pylori isolates in a single patient and underestimation of antimicrobial susceptibility testing. Helicobacter. 2003; 8:202–206.28. Kim YS, Kim N, Kim JM, et al. Helicobacter pylori genotyping findings from multiple cultured isolates and mucosal biopsy specimens: strain diversities of Helicobacter pylori isolates in individual hosts. Eur J Gastroenterol Hepatol. 2009; 21:522–528.29. Rhead JL, Letley DP, Mohammadi M, et al. A new Helicobacter pylori vacuolating cytotoxin determinant, the intermediate region, is associated with gastric cancer. Gastroenterology. 2007; 133:926–936.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Clinical Characteristicsof Patients with Failed Eradication of Helicobacter pylori and Antibiotic Resistance
- Eradication Therapy for Helicobacter pylori with Diagnostic Test for Clarithromycin Resistance
- Antibiotic Resistance in Helicobacter pylori Infection
- The Current Strategy of Helicobacter pylori Eradication
- Metronidazole Resistance and the Eradication of Helicobacter pylori