Yonsei Med J.
2005 Aug;46(4):579-583. 10.3349/ymj.2005.46.4.579.
Evaluation of the Extraction Method for the Cytotoxicity Testing of Latex Gloves
- Affiliations
-
- 1Department of Medical Engineering, Yonsei University College of Medicine, Seoul, Korea. parkjc@yumc.yonsei.ac.kr
- 2Department of Plastic Surgery, Yonsei University College of Medicine, Seoul, Korea.
- 3Department of Laboratory Medicine, Yonsei University College of Medicine, Seoul, Korea.
- 4Yonsei Medical Technology and Quality Evaluation Center, Yonsei University College of Medicine, Seoul, Korea.
- KMID: 1716532
- DOI: http://doi.org/10.3349/ymj.2005.46.4.579
Abstract
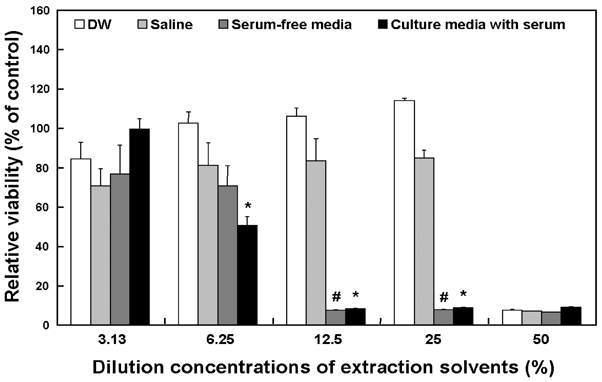
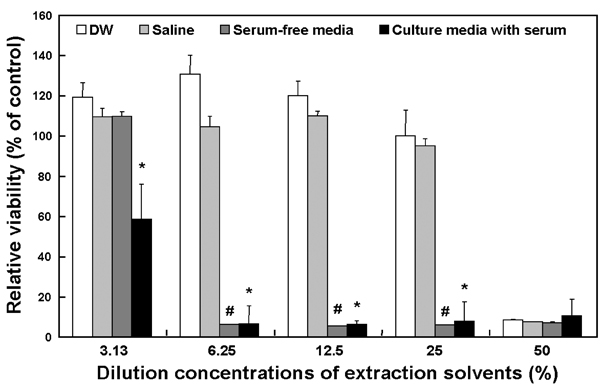
- In this study, the cytotoxicity of medical latex gloves to cultured L-929 cells was determined using various extraction conditions. According to the extraction time and temperature, three types of extraction conditions were used: 1) 24 h at 37 degrees C; 2) 72 h at 37 degrees C; 3) 72 h at 50 degrees C. Also, four different extraction vehicles were used, namely, distilled water (DW), 9 g/l sodium chloride (saline) in DW, and culture media with or without serum. Under the above-mentioned conditions, the samples were extracted and then 2-fold serially diluted in the concentration range 3.13 - 50%. When extracted with either DW or saline for 24 h or 72 h at 37 degrees C, only 50% diluted samples showed distinct cytotoxicity to L-929 cells. Moreover, no cytotoxic potentials were observed when gloves were extracted with DW or saline at 50 degrees C for 72 h. Cytotoxicity was markedly greater when gloves were extracted with culture medium, irrespective of the presence of serum in the medium. These results suggest that optimal extraction conditions should be established for the cytotoxicity evaluations of biomaterials and medical devices.
MeSH Terms
Figure
Reference
-
1. Bollen LS, Svendsen O. Regulatory guidelines for biocompatibility safety testing. Med Plast Biomater. 1997. 05. 16–43.2. Part 10: Method of test for toxicity to cells in culture of extracts from medical devices. BS 5736, Evaluation of medical devices for biological hazards. 1988.3. Biological reactivity tests in vitro. United States Pharmacopeia XXII. 1990.4. Hockley K, Baxter D. Use of 3T3 cell-neutral red uptake assay for irritants as an alternative to the rabbit (Draize) test. Food Chem Toxicol. 1986. 24:473–475.5. Tsuchiya T. Studies on the standardization of cytotoxicity tests and new standard reference materials useful for evaluation the safety of biomaterials. J Biomater Appl. 1994. 9:138–157.6. Shayne CG. Shayne CG, editor. Cytotoxicity testing. Safety evaluation of medical devices. 1997. New York: Marcel Dekker;75–84.7. Ratner BD, Northup SJ. Ratner BD, Hoffman AS, Schoen FJ, Lemons JE, editors. Testing biomaterials. Biomaterials science: an introduction to materials in medicine. 1996. New York: Academic Press;215–220.8. Ciapetti G, Granchi D, Verri E, Savarino L, Cavedagna D, Pizzoferrato A. Application of a combination of neural red and amino black staining for rapid, reliable cytotoxicity testing of biomaterials. Biomaterials. 1996. 17:1259–1264.9. Tsuchiya T, Ikarashi Y, Arai T, Ohhashi J, Isama K, Nakamura A. In vitro tissue/biomaterials toxic responses: correlation with cytotoxic potentials but not cell attachment. Clin Mater. 1994. 16:1–8.10. Part 12: Sample preparation and reference materials. International standard ISO 10993-12, Biological evaluation of medical devices. 1996.11. Part 5: Tests for cytotoxicity: in vitro methods. International standard ISO 10993-5, Biological evaluation of medical devices. 1992.12. Lee JE, Park JC, Park KD, Kim YH, Suh H. In vitro evaluation of PEG modified polyurethanes in cellular toxicity. Biomater Res. 1998. 2:65–68.13. Lee WK, Park KD, Han DK, Suh H, Park JC, Kim YH. Heparinized bovine pericardium as a novel cardiovascular bioprosthesis. Biomaterials. 2000. 21:2323–2330.14. Tsuchiya T, Ikarashi Y, Hata H, Toyoda K, Takahashi M, Uchima T, et al. Comparative studies of the toxicity of standard reference materials in various cytotoxicity tests and in vivo implantation tests. J Appl Biomater. 1993. 4:153–156.15. Kubota Y, Takahashi S, Takahashi I, Patrick G. Different cytotoxic response to gadolinium between mouse and rat alveolar macrophages. Toxicol in Vitro. 2000. 14:309–319.16. Park JC, Park BJ, Lee DH, Suh H, Kim DG, Kwon OH. Evaluation of the cytotoxicity of polyurethane (PU) film containing zinc diethyldithiocarbamate (ZDEC) on various cell lines. Yonsei Med J. 2002. 43:518–526.17. Tsuchiya T, Ikarashi Y, Arai T, Ohhashi J, Nakamura A. Improved sensitivity and decreased sample size in a cytotoxicity test for biomaterials: a modified color microassay using a microplate and crystal violet staining. J Appl Biomater. 1994. 5:361–367.18. Cenni E, Ciapetti G, Granchi D, Arciola CR, Savarino L, Stea S, et al. Established cell lines and primary cultures on testing medical devices in vitro. Toxicol In Vitro. 1999. 13:801–810.19. Liu BS, Yao CH, Chen YS, Hsu SH. In vitro evaluation of degradation and cytotoxicity of a novel composite as a bone substitute. J Biomed Mater Res. 2003. 67:1163–1169.20. Graham DT, Mark GE, Macarthur EB, Pomeroy AR. In vivo validation of a cell culture test for biocompatibility testing of urinary catheters. J Biomed Mater Res. 1984. 18:1125–1135.21. Ruutu M, Alfthan O, Talja M, Andersson C. Cytotoxicity of latex urinary catheters. Br J Urol. 1985. 57:82–87.22. Pariente JL, Bordenave L, Jacob F, Bareille R, Baquey C, Le Guillou M. Cytotoxicity assessment of latex urinary catheters on cultured human urothelial cells. Eur Urol. 2000. 38:640–643.23. section 13, F813-83, Standard practice for direct contact cell culture evaluation of materials for medical devices. Annual book of ASTM standards. 1996.24. Ikarashi Y, Toyoda K, Ohsawa N, Uchima T, Tsuchiya T, Kaniwa M, et al. Comparative studies by cell culture and in vivo implantation test on the toxicity of natural rubber latex materials. J Biomed Mater Res. 1992. 26:339–356.25. Nishi C, Nakajima N, Ikada Y. In vitro evaluation of diepoxy compounds used for biomaterial modification. J Biomed Mater Res. 1995. 29:829–834.