Yonsei Med J.
2005 Aug;46(4):519-525. 10.3349/ymj.2005.46.4.519.
Clinical Significance of p16 Protein Expression Loss and Aberrant p53 Protein Expression in Pancreatic Cancer
- Affiliations
-
- 1Department of Surgery, Yonsei University College of Medicine, Seoul, Korea. yds6110@yumc.yonsei.ac.kr
- 2Department of Pathology, Yonsei University College of Medicine, Seoul, Korea.
- KMID: 1716522
- DOI: http://doi.org/10.3349/ymj.2005.46.4.519
Abstract
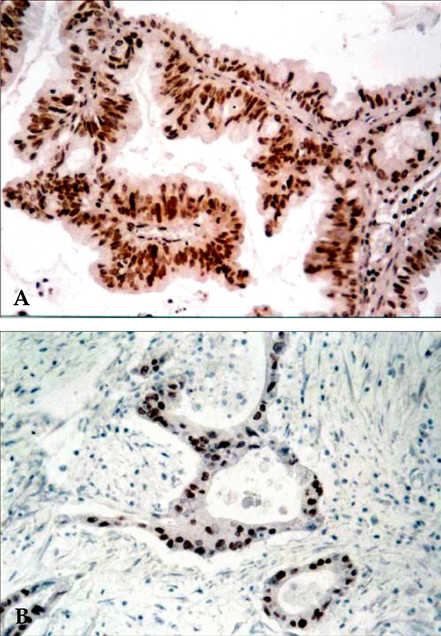
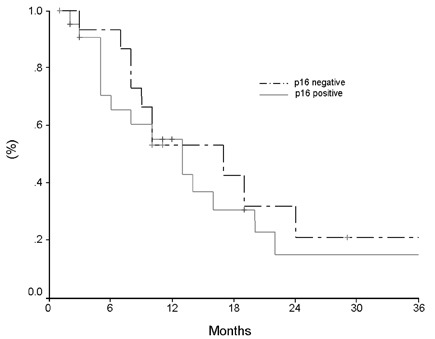
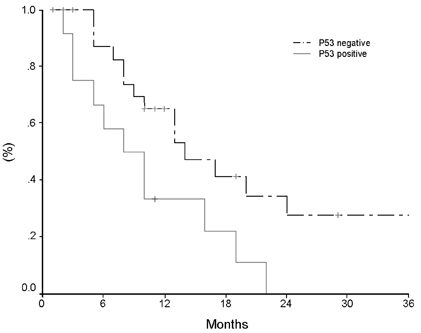
- Pancreatic cancer is a disease with poor prognosis mainly due to low resection rates and late diagnosis. To increase resectability and improve survival rates, a better understanding of pancreatic cancer pathogenesis and more effective screening techniques are required. New methods, such as genetic and molecular alterations, may suggest novel approaches for pancreatic cancer diagnosis and treatment. We immunohistochemically investigated 44 formalin-fixed, paraffin-embedded specimens of pancreatic ductal adenocarcinoma using monoclonal anti-p16 antibodies and monoclonal anti-p53 antibodies. The expressions of p16 and p53 proteins were compared using the Chi-square test with SPSS. Disease-free survival was analyzed using the Kaplan-Meier method, verified by the Log- Rank test. Loss of p16 expression was noted in 20 (45.5%) cases and aberrant p53 protein expression was detected in 14 (31.8%) cases. Loss of p16 expression was associated with a higher incidence of lymph node metastasis (p=0.040) and a more advanced stage (p=0.015), although there was no significant correlation between p16 expression and survival. Aberrant p53 protein expression correlated with histologic grade (p= 0.038). Disease-free survival rate was significantly lower in the aberrant p53 protein positive group compared to the negative group (p=0.029). From our results, we suggest that p53 is not a prognostic factor; however, p16 and p53 genes do play important roles in the progression of pancreatic ductal adenocarcinoma.
Keyword
MeSH Terms
Figure
Cited by 1 articles
-
Prognostic Implications of Cyclin B1, p34cdc2, p27Kip1 and p53 Expression in Gastric Cancer
Dong-Hoon Kim
Yonsei Med J. 2007;48(4):694-700. doi: 10.3349/ymj.2007.48.4.694.
Reference
-
1. Ministry of Health and Welfare, Republic of Korea. Annual Report of the Central Cancer Registry in Korea (1997.1.1~1997.12.31). 1999.2. Yeo CJ, Cameron JL. Pancreatic cancer. Curr Probl Surg. 1999. 36:59–152.3. Crist DW, Cameron JL. The current status of the Whipple operation for periampullary carcinoma. Adv Surg. 1992. 25:21–49.4. Fernandez-del Castillo C, Rattner DW, Warshaw AL. Standards for pancreatic resection in the 1990s. Arch Surg. 1995. 130:295–300.5. Yoon DS, Jeong J, Park YN, Kim KS, Kwon SW, Chi HS, et al. Expression of biliary antigen and its clinical significance in hepatocellular carcinoma. Yonsei Med J. 1999. 40:472–477.6. Caldas C, Hahn SA, da Costa LT, Redston MS, Schutte M, Seymour AB, et al. Frequent somatic mutations and homozygous deletions of the p16 (MTS1) gene in pancreatic adenocarcinoma. Nat Genet. 1994. 8:27–32.7. Redston MS, Caldas C, Seymour AB, Hruban RH, da Costa L, Yeo CJ, et al. p53 mutations in pancreatic carcinoma and evidence of common involvement of homocopolymer tracts in DNA microdeletions. Cancer Res. 1996. 54:3025–3033.8. Goggins M, Schutte M, Lu J, Moskaluk CA, Weinstein CL, Petersen GM, et al. Germline BRCA2 gene mutations in patients with apparently sporadic pancreatic carcinomas. Cancer Res. 1996. 56:5360–5364.9. Rozenblum E, Schutte M, Goggins M, Hahn SA, Panzer S, Zahurak M, et al. Tumor-suppressive pathways in pancreatic carcinoma. Cancer Res. 1997. 57:1731–1734.10. Huang L, Goodrow TL, Zhang S, Klein-Szanto AJP, Chang H, Ruggeri BA. Deletion and mutation analyses of the p16/MTS1 tumor suppressor gene in human ductal pancreatic cancer reveals a higher frequency of abnormalities in tumor derived cell lines than in primary ductal adenocarcinomas. Cancer Res. 1996. 56:1137–1141.11. Hu YX, Watanabe H, Ohtsubo K, Yamaguchi Y, Ha A, Okai T, et al. Frequent loss of p16 expression and its correlation with clinicopathological parameters in pancreatic carcinoma. Clin Cancer Res. 1997. 3:1473–1477.12. Kawesha A, Ghaneh P, Andren-Sandberg A, Ograed D, Skar R, Dawiskiba S, et al. K-ras oncogene subtype mutations are associated with survival but not expression of p53, p16INK4A, p21WAF-1, cyclin D1, erbB-2 and erbB-3 in resected pancreatic ductal adenocarcinoma. Int J Cancer. 2000. 89:469–474.13. Moskaluk CA, Hruban RH, Kern SE. p16 and K-ras gene mutations in the intraductal precursors of human pancreatic adenocarcinoma. Cancer Res. 1997. 57:2140–2143.14. Wilentz RE, Geradts J, Maynard R, Offerhaus GJ, Kang M, Goggins M, et al. Inactivation of the p6 (INK4A) tumor-suppressor gene in pancreatic duct lesions: loss of intranuclear expression. Cancer Res. 1998. 58:4740–4744.15. Bartsch D, Shevlin DW, Callery MP, Norton JP, Wells SA Jr, Goodfellow PJ. Reduced survival inpatients with ductal pancreatic adenocarcinomas associated with CDKN2 mutation. J Natl Cancer Inst. 1996. 88:680–682.16. Eskelinen MJ, Haglund UH. Prognosis of human pancreatic adenocarcinoma: review of clinical and histopathological variables and possible uses of new molecular methods. Eur J Surg. 1999. 165:292–306.17. Sakorafas GH, Tsiotou AG, Tsiotos GG. Molecular biology of pancreatic cancer; oncogenes, tumour suppressor genes, growth factors, and their receptors from a clinical perspective. Cancer Treat Rev. 2000. 26:29–52.18. Dergham ST, Dugan MC, Kucway R, Du W, Kamarauskiene DS, Vaitkevicius VK, et al. Prevalence and clinical significance of combined K-ras mutation and p53 aberration in pancreatic adenocarcinoma. Int J Pancreat. 1997. 21:127–143.19. Ruggeri BA, Huang L, Berger D, Chang H, Klein-Szanto AJ, Goodrow T, et al. Molecular pathology of primary and metastatic ductal pancreatic lesions. Cancer. 1997. 79:700–716.20. Gansauge F, Gansauge S, Schmidt E, Muller J, Beger HG. Prognostic significance of molecular alterations in human pancreatic carcinoma-an immunohistological study. Langenbecks Arch Surg. 1998. 383:152–155.21. Nio Y, Dong M, Iguchi C, Yamasawa K, Toga T, Itakura M, et al. Expression of Bcl-2 and p53 protein inresectable invasive ductal carcinoma of the pancreas: effects on clinical outcome and efficacy of adjuvant chemotherapy. J Surg Oncol. 2001. 76:188–196.22. Nakamori S, Yashima K, Murakami Y, Ishikawa O, Ohigashi H, Imaoka S, et al. Association of p53 gene mutations with short survival in pancreatic adenocarcinoma. Jpn J Cancer Res. 1995. 86:174–181.23. Campani D, Boggi U, Cecchetti D, Esposito I, Ceccarelli F, D'Antonio L, et al. p53 overexpression in lymph node metastases predicts clinical outcome in ductal pancreatic cancer. Pancreas. 1999. 19:26–32.24. Lynch HT, Brand RE, Lynch JF, Fusaro RM, Kern SE. Hereditary factors in pancreatic cancer. J Hepatobiliary Pancreat Surg. 2002. 9:12–31.25. Zhang SY, Ruggeri B, Agarwal P, Sorling AF, Obara T, Ura H, et al. Immunohistochemical analysis of p53 expression in human pancreatic carcinomas. Arch Pathol Lab Med. 1994. 118:150–154.26. Yokoyama M, Yamanaka Y, Friess H, Buchler M, Murray K. P53 expression in human pancreatic cancer correlates with enhanced biological aggressiveness. Anticancer Res. 1994. 14:2477–2483.27. Pancreas Club Meeting. May 19, 1996, San Francisco, California. Am J Surg. 1997. 173:153–158.28. Naumann M, Savitskaia N, Eilert C, Schramm A, Kalthoff H, Schmiegel W. Frequent codeletion of p16/MTS1 and p15/MTS2 and genetic alterations in p16/MTS1 in pancreatic tumors. Gastroenterology. 1996. 110:1215–1224.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Effect of p53 and p16 Protein Expression in Relation to Body Mass Index for Breast Cancer Risk
- Significance of K-ras mutation, K-ras expression and p53 expression in pancreatic cancer
- Expression of p16, Rb and FHIT Proteins in Urothelial Carcinoma of the Urinary Bladder
- Immunohistochemical Analysis of Abnormal p16INK4A Protein Expression in Human Breast Cancer
- The Loss of p16(ink4)Expression is Strongly Associated with Hyperme thylation-Related Inactivation in Breast Carcinoma