Lab Anim Res.
2013 Dec;29(4):204-211. 10.5625/lar.2013.29.4.204.
Protective effect of diallyl disulfide on cyclophosphamide-induced testicular toxicity in rats
- Affiliations
-
- 1College of Veterinary Medicine, Chonnam National University, Gwangju, Korea. toxkim@jnu.ac.kr
- KMID: 1716215
- DOI: http://doi.org/10.5625/lar.2013.29.4.204
Abstract
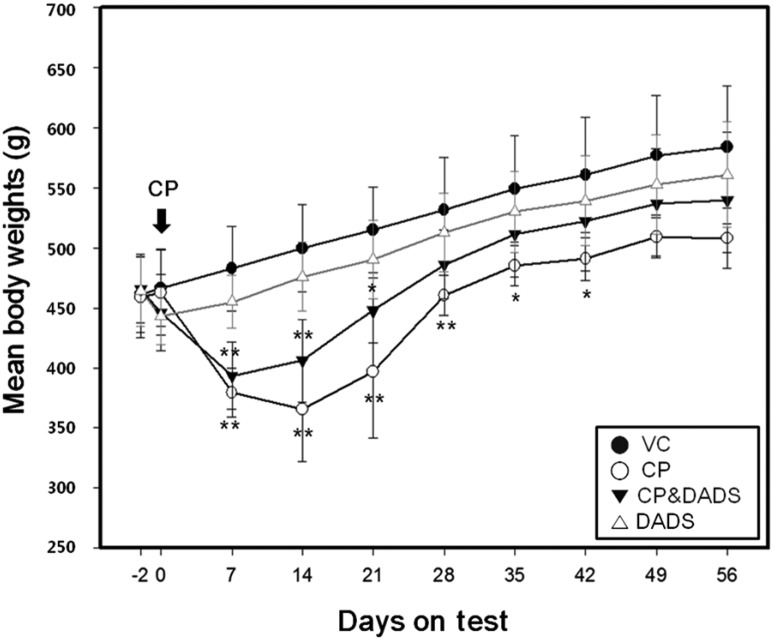
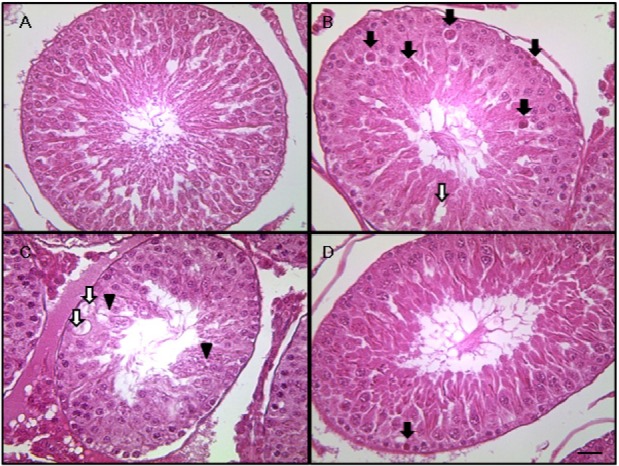
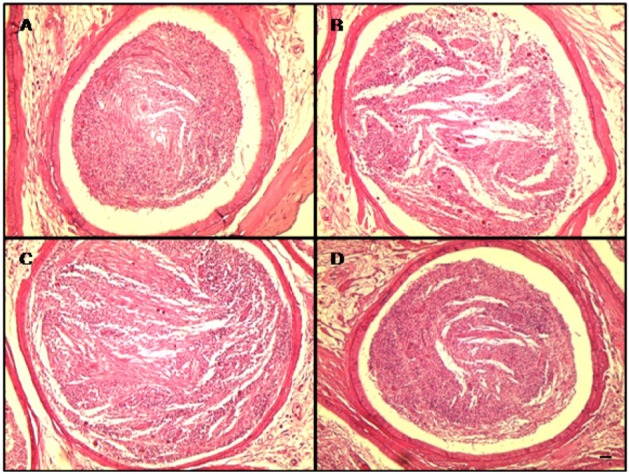
- This study investigated the protective effects of diallyl disulfide (DADS) against cyclophosphamide (CP)-induced testicular toxicity in male rats. DADS was gavaged to rats once daily for 3 days at 100 mg/kg/day. One hour after the final DADS treatment, the rats were given a single intraperitoneal dose of 150 mg/kg CP. All rats were killed and necropsied on day 56 after CP treatment. Parameters of testicular toxicity included reproductive organ weight, testicular sperm head count, epididymal sperm motility and morphology, epididymal index, and histopathologic examinations. The CP treatment caused a decrease in body weight, testicular sperm head count, epididymal sperm motility, and epididymal index. The histopathological examination revealed various morphological alterations, characterized by degeneration of spermatogonia/spermatocytes, vacuolization, and decreased number of spermatids/spermatocytes in the testis, and cell debris and mild oligospermia in the ductus epididymis. In contrast, DADS pretreatment effectively attenuated the testicular toxicity caused by CP, including decreased sperm head count, epididymal sperm motility, and epididymal index and increased histopathological alterations in the testis and epididymis. These results indicate that DADS attenuates testicular toxicity induced by CP in rats.
Keyword
MeSH Terms
Figure
Reference
-
1. Sahin K, Sahin N, Kucuk O. Lycopene and chemotherapy toxicity. Nutr Cancer. 2010; 62(7):988–995. PMID: 20924974.
Article2. Baumann F, Preiss R. Cyclophosphamide and related anticancer drugs. J Chromatogr B Biomed Sci Appl. 2001; 764(1-2):173–192. PMID: 11817027.
Article3. Shanafelt TD, Lin T, Geyer SM, Zent CS, Leung N, Kabat B, Bowen D, Grever MR, Byrd JC, Kay NE. Pentostatin, cyclophosphamide, and rituximab regimen in older patients with chronic lymphocytic leukemia. Cancer. 2007; 109(11):2291–2298. PMID: 17514743.
Article4. Uber WE, Self SE, Van Bakel AB, Pereira NL. Acute antibody-mediated rejection following heart transplantation. Am J Transplant. 2007; 7(9):2064–2074. PMID: 17614978.
Article5. Qureshi MS, Pennington JH, Goldsmith HJ, Cox PE. Cyclophosphamide therapy and sterility. Lancet. 1972; 2(7790):1290–1291. PMID: 4117814.
Article6. Elangovan N, Chiou TJ, Tzeng WF, Chu ST. Cyclophosphamide treatment causes impairment of sperm and its fertilizing ability in mice. Toxicology. 2006; 222(1-2):60–70. PMID: 16517039.
Article7. Rezvanfar M, Sadrkhanlou R, Ahmadi A, Shojaei-Sadee H, Rezvanfar M, Mohammadirad A, Salehnia A, Abdollahi M. Protection of cyclophosphamide-induced toxicity in reproductive tract histology, sperm characteristics, and DNA damage by an herbal source; evidence for role of free-radical toxic stress. Hum Exp Toxicol. 2008; 27(12):901–910. PMID: 19273545.
Article8. Manda K, Bhatia AL. Prophylactic action of melatonin against cyclophosphamide-induced oxidative stress in mice. Cell Biol Toxicol. 2003; 19(6):367–372. PMID: 15015761.
Article9. Bhatia K, Ahmad F, Rashid H, Raisuddin S. Protective effect of S-allylcysteine against cyclophosphamide-induced bladder hemorrhagic cystitis in mice. Food Chem Toxicol. 2008; 46(11):3368–3374. PMID: 18786597.
Article10. Motawi TM, Sadik NA, Refaat A. Cytoprotective effects of DL-alpha-lipoic acid or squalene on cyclophosphamide-induced oxidative injury: an experimental study on rat myocardium, testicles and urinary bladder. Food Chem Toxicol. 2010; 48(8-9):2326–2336. PMID: 20573578.
Article11. Murata M, Suzuki T, Midorikawa K, Oikawa S, Kawanishi S. Oxidative DNA damage induced by a hydroperoxide derivative of cyclophosphamide. Free Radic Biol Med. 2004; 37(6):793–802. PMID: 15304255.
Article12. Kothari S, Thompson A, Agarwal A, du Plessis SS. Free radicals: their beneficial and detrimental effects on sperm function. Indian J Exp Biol. 2010; 48(5):425–435. PMID: 20795359.13. Vernet P, Aitken RJ, Drevet JR. Antioxidant strategies in the epididymis. Mol Cell Endocrinol. 2004; 216(1-2):31–39. PMID: 15109742.
Article14. Ilbey YO, Ozbek E, Simsek A, Otunctemur A, Cekmen M, Somay A. Potential chemoprotective effect of melatonin in cyclophosphamide- and cisplatin-induced testicular damage in rats. Fertil Steril. 2009; 92(3):1124–1132. PMID: 18829000.
Article15. Selvakumar E, Prahalathan C, Mythili Y, Varalakshmi P. Beneficial effects of DL-alpha-lipoic acid on cyclophosphamide-induced oxidative stress in mitochondrial fractions of rat testis. Chem Biol Interact. 2005; 152(1):59–66. PMID: 15766923.16. Nakagawa H, Tsuta K, Kiuchi K, Senzaki H, Tanaka K, Hioki K, Tsubura A. Growth inhibitory effects of diallyl disulfide on human breast cancer cell lines. Carcinogenesis. 2001; 22(6):891–897. PMID: 11375895.
Article17. Guyonnet D, Belloir C, Suschetet M, Siess MH, Le Bon AM. Mechanisms of protection against aflatoxin B(1) genotoxicity in rats treated by organosulfur compounds from garlic. Carcinogenesis. 2002; 23(8):1335–1341. PMID: 12151352.
Article18. Manesh C, Kuttan G. Alleviation of cyclophosphamide-induced urotoxicity by naturally occurring sulphur compounds. J Exp Clin Cancer Res. 2002; 21(4):509–517. PMID: 12636097.19. Pedraza-Chaverrí J, González-Orozco AE, Maldonado PD, Barrera D, Medina-Campos ON, Hernández-Pando R. Diallyl disulfide ameliorates gentamicin-induced oxidative stress and nephropathy in rats. Eur J Pharmacol. 2003; 473(1):71–78. PMID: 12877940.
Article20. Fukao T, Hosono T, Misawa S, Seki T, Ariga T. The effects of allyl sulfides on the induction of phase II detoxification enzymes and liver injury by carbon tetrachloride. Food Chem Toxicol. 2004; 42(5):743–749. PMID: 15046820.
Article21. Singh SV, Pan SS, Srivastava SK, Xia H, Hu X, Zaren HA, Orchard JL. Differential induction of NAD(P)H:quinone oxidoreductase by anti-carcinogenic organosulfides from garlic. Biochem Biophys Res Commun. 1998; 244(3):917–920. PMID: 9535768.
Article22. Guyonnet D, Siess MH, Le Bon AM, Suschetet M. Modulation of phase II enzymes by organosulfur compounds from allium vegetables in rat tissues. Toxicol Appl Pharmacol. 1999; 154(1):50–58. PMID: 9882591.
Article23. Wu CC, Sheen LY, Chen HW, Tsai SJ, Lii CK. Effects of organosulfur compounds from garlic oil on the antioxidation system in rat liver and red blood cells. Food Chem Toxicol. 2001; 39(6):563–569. PMID: 11346486.
Article24. Matsui H, Mitsumori K, Yasuhara K, Onodera H, Shimo T, Takahashi M. Morphological evaluation of cyclophosphamide testicular toxicity in rats using quantitative morphometry of spermatogenic cycle stages. J Toxicol Sci. 1995; 20(4):407–414. PMID: 8531236.
Article25. Abraham P, Indirani K, Sugumar E. Effect of cyclophosphamide treatment on selected lysosomal enzymes in the kidney of rats. Exp Toxicol Pathol. 2007; 59(2):143–149. PMID: 17686619.
Article26. Ayhanci A, Yaman S, Sahinturk V, Uyar R, Bayramoglu G, Senturk H, Altuner Y, Appak S, Gunes S. Protective effect of seleno-L-methionine on cyclophosphamide-induced urinary bladder toxicity in rats. Biol Trace Elem Res. 2010; 134(1):98–108. PMID: 19629405.
Article27. Wu CC, Sheen LY, Chen HW, Kuo WW, Tsai SJ, Lii CK. Differential effects of garlic oil and its three major organosulfur components on the hepatic detoxification system in rats. J Agric Food Chem. 2002; 50(2):378–383. PMID: 11782211.
Article28. Kim KH, Shin IS, Lim JH, Kim SH, Park NH, Moon C, Kim SH, Shin DH, Kim JC. Dose-response effects of epichlorohydrin on male reproductive function in rats. Toxicol Res. 2009; 25:203–207.
Article29. Johnson FE, Farr SA, Mawad M, Woo YC. Testicular cytotoxicity of intravenous methotrexate in rats. J Surg Oncol. 1994; 55(3):175–178. PMID: 8176928.
Article30. Tates AD. Validation studies with the micronucleus test for early spermatids of rats. A tool for detecting clastogenicity of chemicals in differentiating spermatogonia and spermatocytes. Mutagenesis. 1992; 7(6):411–419. PMID: 1474916.
Article31. Cai L, Hales BF, Robaire B. Induction of apoptosis in the germ cells of adult male rats after exposure to cyclophosphamide. Biol Reprod. 1997; 56(6):1490–1497. PMID: 9166702.32. Meistrich ML, Parchuri N, Wilson G, Kurdoglu B, Kangasniemi M. Hormonal protection from cyclophosphamide-induced inactivation of rat stem spermatogonia. J Androl. 1995; 16(4):334–341. PMID: 8537251.33. Kaur F, Sangha GK, Bilaspuri GS. Cyclophosphamide-induced structural and biochemical changes in testis and epididymidis of rats. Indian J Exp Biol. 1997; 35(7):771–775. PMID: 9418379.34. Lear L, Nation RL, Stupans I. Effects of cyclophosphamide and adriamycin on rat hepatic microsomal glucuronidation and lipid peroxidation. Biochem Pharmacol. 1992; 44(4):747–753. PMID: 1510722.
Article35. Haenen GR, Vermeulen NP, Tai Tin Tsoi JN, Ragetli HM, Timmerman H, Blast A. Activation of the microsomal glutathione-S-transferase and reduction of the glutathione dependent protection against lipid peroxidation by acrolein. Biochem Pharmacol. 1988; 37(10):1933–1938. PMID: 3377801.
Article36. Mythili Y, Sudharsan PT, Selvakumar E, Varalakshmi P. Protective effect of DL-alpha-lipoic acid on cyclophosphamide induced oxidative cardiac injury. Chem Biol Interact. 2004; 151(1):13–19. PMID: 15607758.37. Aitken RJ, Harkiss D, Buckingham D. Relationship between iron-catalysed lipid peroxidation potential and human sperm function. J Reprod Fertil. 1993; 98(1):257–265. PMID: 8345470.
Article38. Alvarez JG, Storey BT. Differential incorporation of fatty acids into and peroxidative loss of fatty acids from phospholipids of human spermatozoa. Mol Reprod Dev. 1995; 42(3):334–346. PMID: 8579848.
Article39. Codrington AM, Hales BF, Robaire B. Exposure of male rats to cyclophosphamide alters the chromatin structure and basic proteome in spermatozoa. Hum Reprod. 2007; 22(5):1431–1442. PMID: 17303633.
Article40. Unnikrishnan MC, Soudamini KK, Kuttan R. Chemoprotection of garlic extract toward cyclophosphamide toxicity in mice. Nutr Cancer. 1990; 13(3):201–207. PMID: 2308875.
Article41. Ola-Mudathir KF, Suru SM, Fafunso MA, Obioha UE, Faremi TY. Protective roles of onion and garlic extracts on cadmium-induced changes in sperm characteristics and testicular oxidative damage in rats. Food Chem Toxicol. 2008; 46(12):3604–3611. PMID: 18824205.
Article42. Sadik NA. Effects of diallyl sulfide and zinc on testicular steroidogenesis in cadmium-treated male rats. J Biochem Mol Toxicol. 2008; 22(5):345–353. PMID: 18972399.
Article43. Meistrich ML. Evaluation of reproductive toxicity by testicular sperm head counts. J Am Coll Toxicol. 1989; 8:551–567.
Article44. Schimenti KJ, Hanneman WH, Schimenti JC. Evidence for cyclophosphamide-induced gene conversion and mutation in mouse germ cells. Toxicol Appl Pharmacol. 1997; 147(2):343–350. PMID: 9439729.
Article45. Aguilar-Mahecha A, Hales BF, Robaire B. Effects of acute and chronic cyclophosphamide treatment on meiotic progression and the induction of DNA double-strand breaks in rat spermatocytes. Biol Reprod. 2005; 72(6):1297–1304. PMID: 15673603.46. Kasuga S, Uda N, Kyo E, Ushijima M, Morihara N, Itakura Y. Pharmacologic activities of aged garlic extract in comparison with other garlic preparations. J Nutr. 2001; 131(3s):1080S–1084S. PMID: 11238821.
Article47. Ponnusamy M, Pari L. Protective role of diallyl tetrasulfide on cadmium-induced testicular damage in adult rats: a biochemical and histological study. Toxicol Ind Health. 2011; 27(5):407–416. PMID: 21245201.
Article48. Sheen LY, Sheu SF, Tsai SJ, Meng RH, Lii CK. Effect of garlic active principle, diallyl disulfide, on cell viability, lipid peroxidation, glutathione concentration and its related enzyme activities in primary rat hepatocytes. Am J Chin Med. 1999; 27(1):95–105. PMID: 10354821.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Diallyl disulfide attenuates acetaminophen-induced renal injury in rats
- Exploring the role and mechanisms of diallyl trisulfide and diallyl disulfide in chronic constriction-induced neuropathic pain in rats
- Diallyl Disulfide Prevents Cyclophosphamide-Induced Hemorrhagic Cystitis in Rats through the Inhibition of Oxidative Damage, MAPKs, and NF-kappaB Pathways
- The Effect of Garlic Derivatives (S-Allylmercaptocysteine, Diallyl Disulfide, and S-Allylcysteine) on Gentamicin Induced Ototoxicity: An Experimental Study
- Age-dependent Effect of Metabolism and Testicular Toxicity to di(2-ethylhexyl) Phthalate