J Korean Med Sci.
2007 Oct;22(5):891-897. 10.3346/jkms.2007.22.5.891.
15-Deoxy-delta 12,14-ProstaglandinJ2 Regulates Dedifferentiation through Peroxisome Proliferator-Activated Receptor-gamma-Dependent Pathway but Not COX-2 Expression in Articular Chondrocytes
- Affiliations
-
- 1Department of Biological Sciences, College of Natural Sciences, Kongju National University, Gongju, Korea. ksj85@kongju.ac.kr
- 2Department of Biology, College of Natural Sciences, Kyungpook National University, Daegu, Korea.
- KMID: 1713299
- DOI: http://doi.org/10.3346/jkms.2007.22.5.891
Abstract
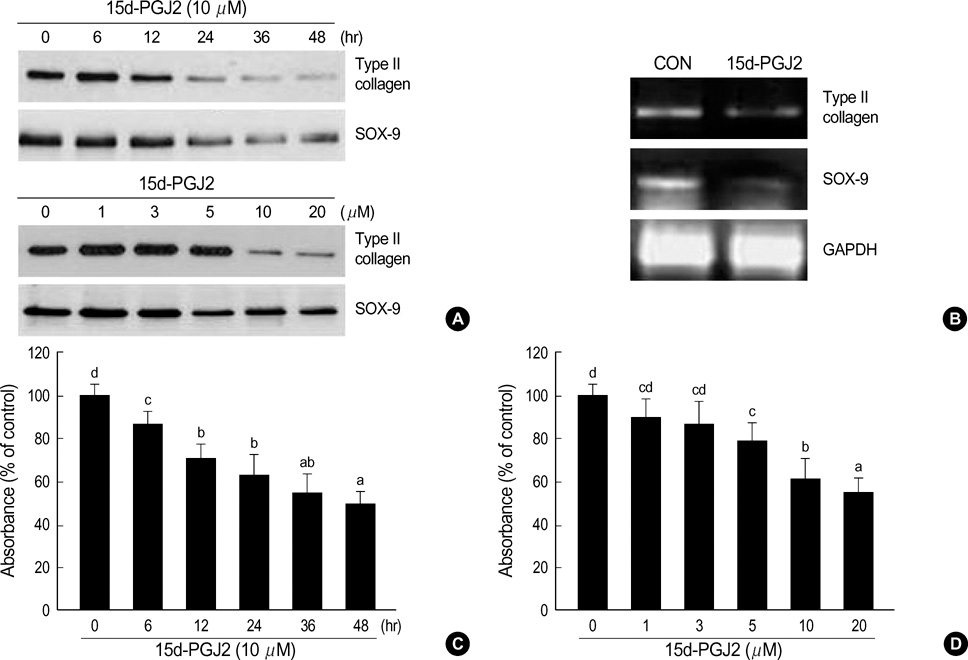
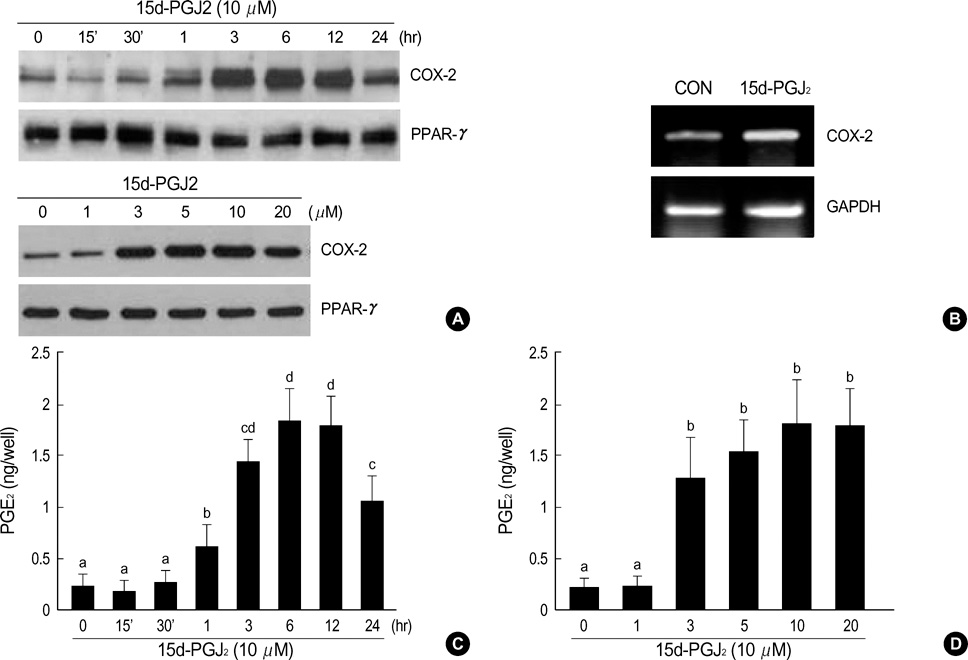
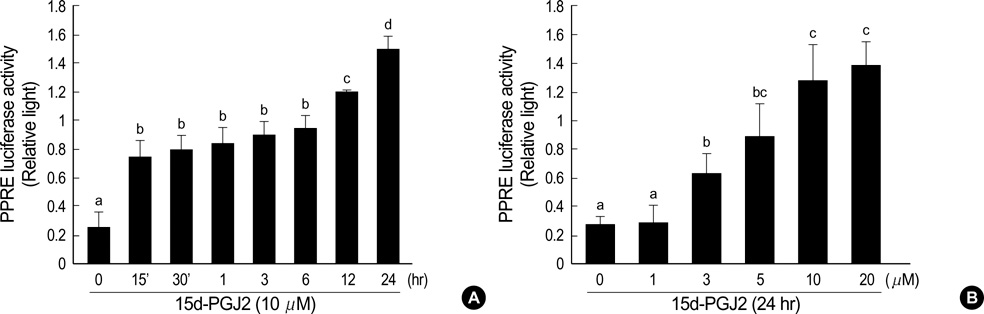
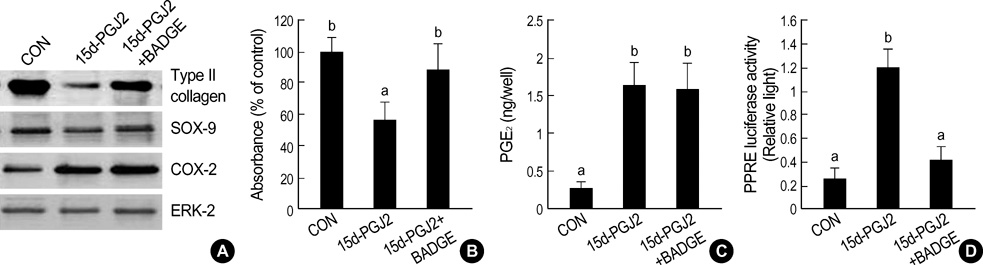
- Peroxisome proliferator-activated receptors-gamma (PPAR-gamma) is critical for phenotype determination at early differentiation stages of mesenchymal cells, whereas its physiological role is unclear. Therefore, we investigated the role of 15-deoxy-delta 12,14-prostaglandinJ2 (15d-PGJ2), the natural receptor ligand for PPAR-gamma, on dedifferentiation and inflammatory responses, such as COX-2 expression and PGE2 production, in articular chondrocytes. Our data indicate that the 15d-PGJ2 caused a loss of differentiated chondrocyte phenotype as demonstrated by inhibition of type II collagen and proteoglycan synthesis. 15d-PGJ2 also induced COX-2 expression and PGE2 production. The 15d-PGJ2-induced dedifferentiation effect seems to be dependent on PPAR-gamma activation, as the PPRE luciferase activity increased and PPAR-gamma antagonist, BADGE, abolished type II collagen expression. However, BADGE did not block 15d-PGJ2-induced COX-2 expression. Collectively, our findings suggest that PPAR-gamma-dependent and -independent mechanisms of 15d-PGJ2-induced dedifferentiation and inflammatory responses in articular chondrocytes, respectively. Additionally, these data suggest that targeted modulation of the PPAR-gamma pathway may offer a novel approach for therapeutic inhibition of joint tissue degradation.
MeSH Terms
-
Animals
Arteries/*metabolism
Cell Differentiation
Chondrocytes/*metabolism
Cyclooxygenase 2/*metabolism
Dinoprostone/metabolism
Dose-Response Relationship, Drug
*Gene Expression Regulation, Enzymologic
Genes, Reporter
Immunoblotting
PPAR gamma/*metabolism
Prostaglandin D2/*analogs & derivatives/metabolism
Rabbits
Time Factors
Transfection
Figure
Reference
-
1. Tontonoz P, Hu E, Spiegelman BM. Stimulation of adipogenesis in fibroblasts by PPAR gamma 2, a lipid-activated transcription factor. Cell. 1994. 79:1147–1156.2. Chawla A, Lazar M. Peroxisome proliferator and retinoid signaling pathways co-regulate preadipocyte phenotype and survival. Proc Natl Acad Sci USA. 1994. 91:1786–1790.
Article3. Kliewer SA, Forman BM, Blumberg B, Ong ES, Borgmeyer U, Mangelsdorf DJ, Umesono K, Evans RM. Differential expression and activation of a family of murine peroxisome proliferator-activated receptors. Proc Natl Acad Sci USA. 1994. 91:7355–7359.
Article4. Amri EZ, Bonino F, Ailhaud G, Abumrad NA, Grimaldi PA. Cloning of a protein that mediates transcriptional effects of fatty acids in preadipocytes. Homology to peroxisome proliferator-activated receptors. J Biol Chem. 1995. 270:2367–2371.5. Schmidt A, Endo N, Rutledge SJ, Vogel R, Shinar D, Rodan GA. Identification of a new member of the steroid hormone receptor superfamily that is activated by a peroxisome proliferator and fatty acids. Mol Endocrinol. 1992. 6:1634–1641.
Article6. Chawla A, Schwarz EJ, Dimaculangan DD, Lazar MA. Peroxisome proliferator-activated receptor (PPAR) gamma: adipose-predominant expression and induction early in adipocyte differentiation. Endocrinology. 1994. 135:798–800.
Article7. Lin FT, Lane MD. CCAAT/enhancer binding protein alpha is sufficient to initiate the 3T3-L1 adipocyte differentiation program. Proc Natl Acad Sci USA. 1994. 91:8757–8761.
Article8. Clark RB, Bishop-Bailey D, Estrada-Hernandez T, Hla T, Puddington L, Padula SJ. The nuclear receptor PPAR gamma and immunoregulation: PPAR gamma mediates inhibition of helper T cell responses. J Immunol. 2000. 164:1364–1371.9. Bordji K, Grillasca JP, Gouze JN, Magdalou J, Schohn H, Keller JM, Bianchi A, Dauca M, Netter P, Terlain B. Evidence for the presence of peroxisome proliferator-activated receptor (PPAR) alpha and gamma and retinoid Z receptor in cartilage. PPARgamma activation modulates the effects of interleukin-1beta on rat chondrocytes. J Biol Chem. 2000. 275:12243–12250.10. Fahmi H, Di Battista JA, Pelletier JP, Mineau F, Ranger P, Martel-Pelletier J. Peroxisome proliferator--activated receptor gamma activators inhibit interleukin-1beta-induced nitric oxide and matrix metalloproteinase 13 production in human chondrocytes. J Arthritis Rheum. 2001. 44:595–607.11. Kliewer SA, Umesono K, Noonan DJ, Heyman RA, Evans RM. Convergence of 9-cis retinoic acid and peroxisome proliferator signalling pathways through heterodimer formation of their receptors. Nature. 1992. 358:771–774.
Article12. Fajas L, Debril MB, Auwerx J. Peroxisome proliferators-activated receptor-gamma: from adipogenesis to carcinogenesis. J Mol Endocrinol. 2001. 27:1–9.13. Forman BM, Tontonoz P, Chen J, Brun RP, Spiegelman BM, Evans RM. 15-Deoxy-delta 12, 14-prostaglandin J2 is a ligand for the adipocyte determination factor PPAR gamma. Cell. 1995. 83:803–812.14. Ricote M, Li AC, Willson TM, Kelly CJ, Glass CK. The peroxisome proliferator-activated receptor-gamma is a negative regulator of macrophage activation. Nature. 1998. 391:79–82.15. Sarraf P, Mueller E, Jones D, King FJ, DeAngelo DJ, Partridge JB, Holden SA, Chen LB, Singer S, Fletcher C, Spiegelman BM. Differentiation and reversal of malignant changes in colon cancer through PPARgamma. Nat Med. 1998. 4:1046–1052.16. Tontonoz P, Singer S, Forman BM, Sarraf P, Fletcher JA, Fletcher CD, Brun RP, Mueller E, Altiok S, Oppenheim H, Evans RM, Spiegelman BM. Terminal differentiation of human liposarcoma cells induced by ligands for peroxisome proliferator-activated receptor gamma and the retinoid X receptor. Proc Natl Acad Sci USA. 1997. 94:237–241.17. Mukherjee R, Davies PJ, Crombie DJ, Bischoff ED, Cesario RM, Jow L, Hamann LG, Boehm MF, Mondon CE, Nadzan AM, Paterniti JR Jr, Heyman RA. Sensitization of diabetic and obese mice to insulin by retinoid X receptor agonists. Nature. 1997. 386:407–410.
Article18. DeLise AM, Fischer L, Tuan RS. Cellular interactions and signaling in cartilage development. Osteoarthritis Cartilage. 2000. 8:309–334.
Article19. Sandell LJ, Aigner T. Articular cartilage and changes in arthritis. An introduction: cell biology of osteoarthritis. Arthritis Res. 2001. 3:107–113.
Article20. Arend WP, Dayer JM. Inhibition of the production and effects of interleukin-1 and tumor necrosis factor alpha in rheumatoid arthritis. Arthritis Rheum. 1995. 38:151–160.21. Lotz M, Blanco FJ, von Kempis J, Dudler J, Maier R, Villiger PM, Geng Y. Cytokine regulation of chondrocyte functions. J Rheumatol Suppl. 1995. 43:104–108.22. Dingle JT. Cartilage maintenance in osteoarthritis: interaction of cytokines, NSAID and prostaglandins in articular cartilage damage and repair. J Rheumatol Suppl. 1991. 28:30–37.23. Yoon YM, Kim SJ, Oh CD, Ju JW, Song WK, Yoo YJ, Huh TL, Chun JS. Maintenance of differentiated phenotype of articular chondrocytes by protein kinase C and extracellular signal-regulated protein kinase. J Biol Chem. 2002. 277:8412–8420.
Article24. SAS Institute. SAS/STAT User's Guide. 1989. Version 6. Cary, NC, USA: SAS Institute.25. Goldring MB, Birkhead J, Sandell LJ, Kimura T, Krane SM. Interleukin 1 suppresses expression of cartilage-specific types II and IX collagens and increases types I and III collagens in human chondrocytes. J Clin Invest. 1988. 82:2026–2037.
Article26. Kim SJ, Ju JW, Oh CD, Yoon YM, Song WK, Kim JH, Yoo YJ, Bang OS, Kang SS, Chun JS. ERK-1/2 and p38 kinase oppositely regulate nitric oxide-induced apoptosis of chondrocytes in association with p53, caspase-3, and differentiation status. J Biol Chem. 2002. 277:1332–1339.
Article27. Gay S, Gay RE, Koopman WJ. Molecular and cellular mechanisms of joint destruction in rheumatoid arthritis: two cellular mechanisms explain joint destruction? Ann Rheum Dis. 1993. 52:Suppl 1. 39–47.
Article28. Tsubouchi Y, Kawahito Y, Kohno M, Inoue K, Hla T, Sano H. Feedback control of the arachidonate cascade in rheumatoid synoviocytes by 15d-PGJ2. Biochem Biophys Res Commun. 2001. 283:750–755.29. Shibata T, Kondo M, Osawa T, Shibata N, Kobayashi M, Uchida K. 15-deoxy-delta 12,14-prostaglandin J2. A prostaglandin D2 metabolite generated during inflammatory processes. J Biol Chem. 2002. 277:10459–10466.30. Shan ZZ, Masuko-Hongo K, Dai SM, Nakamura H, Kato T, Nishioka K. A potential role of 15-deoxy-delta-postaglandin J2 for induction of human articular chondrocyte apoptosis in arthritis. J Biol Chem. 2004. 279:37939–37950.31. Glass CK, Rosenfeld MG. The coregulator exchange in transcriptional functions of nuclear receptors. Genes Dev. 2000. 14:121–141.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- ERK-1/-2 and p38 Kinase Oppositely Regulate 15-deoxy-delta(12,14)-prostaglandinJ2-Induced PPAR-gamma Activation That Mediates Dedifferentiation But Not Cyclooxygenase-2 Expression in Articular Chondrocytes
- Src Kinase Regulates Nitric Oxide-induced Dedifferentiation and Cyclooxygenase-2 Expression in Articular Chondrocytes via p38 Kinase-dependent Pathway
- Peroxisome Proliferator Activated Receptor-delta (PPAR-delta)
- 2-Deoxy-D-glucose regulates dedifferentiation through beta-catenin pathway in rabbit articular chondrocytes
- Antineoplastic effect of endogenous peroxisome proliferator-activated receptor gamma ligand, 15-deoxy-delta(12,14)-prostaglandin J2, on cholangiocarcinoma cells