J Korean Med Sci.
2005 Aug;20(4):591-597. 10.3346/jkms.2005.20.4.591.
Comparison of Clinical Efficacy of Newfactan(R) versus Surfacten(R) for the Treatment of Respiratory Distress Syndrome in the Newborn Infants
- Affiliations
-
- 1Department of Pediatrics, Samsung Seoul Hospital, Samsung Cheil Hospital, Korea. wspark@smc.samsung.co.kr
- 2Kangbuk Samsung Hospital, Sungkyunkwan University School of Medicine, Korea.
- 3Kangnam Cha Hospital, Pochon Cha University College of Medicine, Seoul, Korea.
- 4Soonchunhyang University Bucheon Hospital, Soonchunhyang University College of Medicine, Bucheon, Korea.
- KMID: 1712738
- DOI: http://doi.org/10.3346/jkms.2005.20.4.591
Abstract
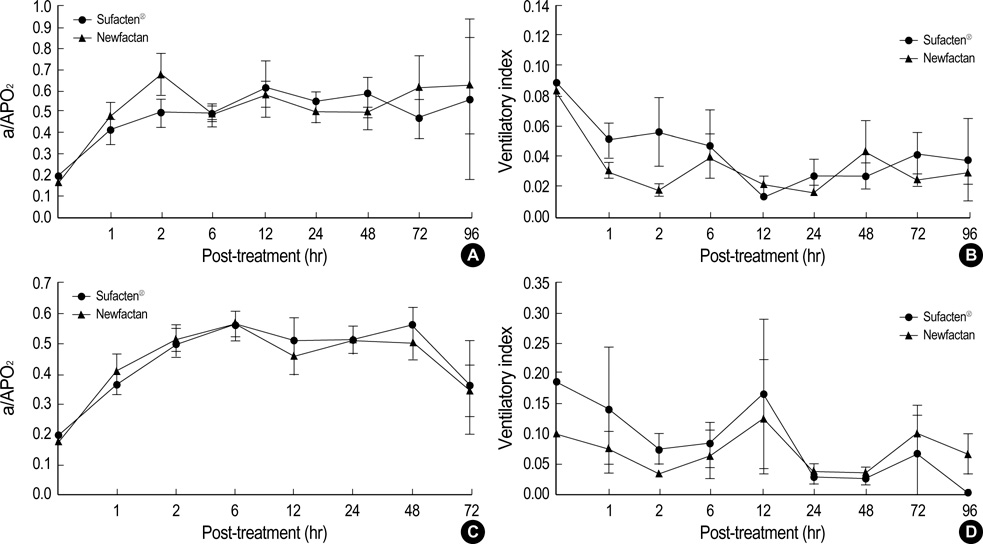
- Newfactan(R) is a domestically developed, bovine lung-derived, semi-synthetic surfactant. The aim of this study was to compare the clinical efficacy of Newfactan(R) with that of Surfacten(R) in the treatment of respiratory distress syndrome (RDS). Newfactan(R) or Surfacten(R) was randomly allocated to 492 newborn infants who were diagnosed as RDS and required surfactant instillation in four participating hospitals. The comparisons were made individually in two subsets of infants by birth weight (<1,500 g group [n=253] and >or=1,500 g group [n=239]). Short-term responses to surfactant and acute complications, such as the total doses of surfactant instilled, response type, extubation rate, ventilator settings, changes in respiratory parameters, air leak, patent ductus arteriosus, pulmonary hemorrhage, and intraventricular hemorrhage, and mortality during the 96 hr after surfactant instillation were measured. Long-term outcome and complications, such as total duration of intubation, bronchopulmonary dysplasia and periventricular leukomalacia, and ultimate mortality were measured. There were no significant differences in demographic and perinatal variables, shortterm responses to surfactant and acute complications, and long-term outcome and complications between Newfactan(R) and Surfacten(R) in both birth weight groups. We concluded that Newfactan(R) was comparable to Surfacten(R) in the clinical efficacy in the treatment of RDS in both birth weight groups.
MeSH Terms
Figure
Cited by 1 articles
-
Early Prophylactic Use of Curosurf® Versus Newfactan® for Respiratory Distress Syndrome in Premature Infants
Young Joo Na, Jang Hoon Lee, Moon Sung Park
Perinatology. 2016;27(3):162-167. doi: 10.14734/PN.2016.27.3.162.
Reference
-
1. Rodriguez RJ. Management of respiratory distress syndrome: an update. Respir Care. 2003. 48:279–286.2. Namgung R, Lee C, Suh JS, Park KI, Han DG. Exogenous surfactant replacement therapy of hyaline membrane disease in premature infants. Yonsei Med J. 1989. 30:355–366.
Article3. Bae CW, Kwon YD, Ko SJ, Kim KS, Kim HM, Park WS, Byun SH, Son CS, Ahn HS, Lee SG, Chang YP, Chung YJ. Surfactant replacement therapy in neonates with respiratory distress syndrome: a collective evaluation of trials from 16 hospitals. J Korean Pediatr Soc. 1993. 36:244–265.4. Bae CW. Surfactant therapy: history and future. The second ChoongWae surfactant symposium program book. 2004. Seoul: Choong-Wae Pharma Corporation;15–24.5. Bae CW, Choi YM, Lee SC, Kang JH, Kim KL, Hahm KS. Comparison of in vitro surface physical properties of four artificial exogeneous pulmonary surfactant preparations. J Korean Pediatr Soc. 1999. 42:1215–1223.6. Lee C, Park MS, Kim JN, Lee JW, You KH, Kwag WJ, Park KI, Gung RN, Han DG. Physical and biological activity of domestic product of modified bovine lung surfactant. J Korean Pediatr Soc. 1997. 40:771–785.7. Lee C, Kim JN, Park MS, Yoon SW, Chang W, Namgung R, Park KI, Han DG. Clinical trial of domestically developed bovine lung surfactant YY-38 in neonatal respiratory distress syndrome. J Korean Pediatr Soc. 1999. 42:472–483.8. Lee JJ, Lee YK, Yoon HS, Park JD, Kim AR, Kim KS, Kim BI, Pi SY. Comparison of surfacten versus newfactan for the treatment of respiratory distress syndrome. J Korean Soc Neonatol. 2000. 7:24–32.9. Ha SH, Baek YW. Clinical effects of newfactan in the treatment of moderate to severe respiratory distress syndrome. J Korean Soc Neonatol. 2001. 8:65–71.10. Hallman M, Merritt TA, Jarvenpaa AL, Boynton B, Mannino F, Gluck L, Moore T, Edwards D. Exogenous human surfactant for treatment of severe respiratory distress syndrome: a randomized prospective clinical trial. J Pediatr. 1985. 106:963–969.
Article11. Konishi M, Chida S, Shimada S, Kasai T, Murakami Y, Cho K, Fujii Y, Maeta H, Fujiwara T. Surfactant replacement therapy in premature babies with respiratory distress syndrome: factors affecting the response to surfactant and comparison of outcome from 1982-86 and 1987-91. Acta Paediatr Jpn. 1992. 34:617–630.
Article12. Jobe AH, Bancalari E. Bronchopulmonary dysplasia. Am J Respir Crit Care Med. 2001. 163:1723–1729.
Article13. Rodriguez RJ, Martin RJ, Fanaroff AA. Fanaroff AA, Martin RJ, editors. The respiratory distress syndrome and its management. Neonatal-perinatal medicine. 2002. 7th ed. St. Louis: Mosby-Year Book.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Comparison of in vitro Surface Physical Properties of Four Artificial Exogenous Pulmonary Surfactant Preparations
- Comparisons of Physical and Biological Activities between Newfactan(R), Surfacten(R) and Exosurf(R)
- Clinical Effects of Newfactan in the Treatment of Moderate to Severe Respiratory Distress Syndrome
- Comparison of Surfacten Versus Newfacten for the Treatment of Respiratory Distress Syndrome
- Comparison of the Therapeutic Effects of Curosurf(R) and Newfactan(R) in Respiratory Distress Syndrome