J Vet Sci.
2013 Dec;14(4):481-486. 10.4142/jvs.2013.14.4.481.
Evaluation of a side population of canine lymphoma cells using Hoechst 33342 dye
- Affiliations
-
- 1The Laboratory of Clinical Pathology and Research Institute for Veterinary Science, College of Veterinary Medicine, Seoul National University, Seoul 151-742, Korea. yongbaek@snu.ac.kr
- 2Department of Population Health and Pathobiology, College of Veterinary Medicine, North Carolina State University, Raleigh, NC 27607, USA.
- 3Department of Clinical Sciences, College of Veterinary Medicine, North Carolina State University, Raleigh, NC 27607, USA.
- KMID: 1712316
- DOI: http://doi.org/10.4142/jvs.2013.14.4.481
Abstract
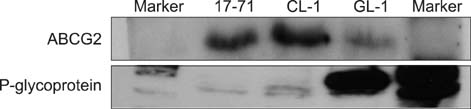
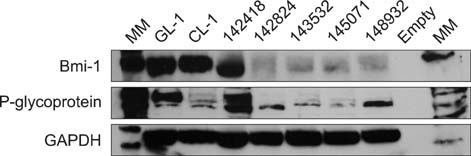
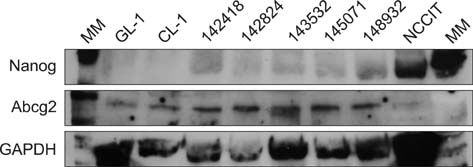
- Cancer stem cell (CSC) research has increased exponentially to gain further insight into the mechanisms underlying both carcinogenesis and chemotherapy resistance. The present study was performed to explore the potential value of a side population (SP) assay for identifying and characterizing putative CSCs among canine lymphoma cells. Canine lymphoma cells from cell lines and clinical samples were subjected to the SP assay consisting of Hoechst 33342 staining and subsequent flow cytometric analysis. The SP assay revealed various amounts of a SP fraction among the canine lymphoma cells. The percentages of SP were not affected by inhibitors of membrane transporters, verapamil hydrochloride, or fumitremorgin C. Most of the canine lymphoma cells expressed high levels of Bmi-1 and membrane transporter proteins such as ABCG2 and phosphorylated (p)-glycoprotein. This investigation lays the groundwork for further studies of the biological behaviors and molecular characteristics of CSCs in cases of canine lymphoma.
Keyword
MeSH Terms
-
Animals
Benzimidazoles/*metabolism
Cell Line, Tumor
Dog Diseases/*diagnosis/drug therapy/pathology
Dogs
Flow Cytometry/*methods/veterinary
Fluorescent Dyes/*metabolism
Gene Expression Regulation, Developmental
Lymphoma/diagnosis/drug therapy/pathology/*veterinary
Neoplastic Stem Cells/drug effects/*metabolism/pathology
Side-Population Cells/drug effects/*metabolism/pathology
Benzimidazoles
Fluorescent Dyes
Figure
Reference
-
1. Goodell MA, Brose K, Paradis G, Conner AS, Mulligan RC. Isolation and functional properties of murine hematopoietic stem cells that are replicating in vivo. J Exp Med. 1996; 183:1797–1806.
Article2. Greenlee PG, Filippa DA, Quimby FW, Patnaik AK, Calvano SE, Matus RE, Kimmel M, Hurvitz AI, Lieberman PH. Lymphomas in dogs: a morphologic, immunologic, and clinical study. Cancer. 1990; 66:480–490.
Article3. Haraguchi N, Utsunomiya T, Inoue H, Tanaka F, Mimori K, Barnard GF, Mori M. Characterization of a side population of cancer cells from human gastrointestinal system. Stem Cells. 2006; 24:506–513.
Article4. Harvey RC, Mullighan CG, Wang X, Dobbin KK, Davidson GS, Bedrick EJ, Chen IM, Atlas SR, Kang H, Ar K, Wilson CS, Wharton W, Murphy M, Devidas M, Carroll AJ, Borowitz MJ, Bowman WP, Downing JR, Relling M, Yang J, Bhojwani D, Carroll WL, Camitta B, Reaman GH, Smith M, Hunger SP, Willman CL. Identification of novel cluster groups in pediatric high-risk B-precursor acute lymphoblastic leukemia with gene expression profiling: correlation with genome-wide DNA copy number alterations, clinical characteristics, and outcome. Blood. 2010; 116:4874–4884.
Article5. Kai K, D'Costa S, Yoon BI, Brody AR, Sills RC, Kim Y. Characterization of side population cells in human malignant mesothelioma cell lines. Lung Cancer. 2010; 70:146–151.
Article6. Kim M, Turnquist H, Jackson J, Sgagias M, Yan Y, Gong M, Dean M, Sharp JG, Cowan K. The multidrug resistance transporter ABCG2 (breast cancer resistance protein 1) effluxes Hoechst 33342 and is overexpressed in hematopoietic stem cells. Clin Cancer Res. 2002; 8:22–28.7. Kiupel M, Teske E, Bostock D. Prognostic factors for treated canine malignant lymphoma. Vet Pathol. 1999; 36:292–300.
Article8. Levine RL. Inherited susceptibility to pediatric acute lymphoblastic leukemia. Nat Genet. 2009; 41:957–958.
Article9. Lobo NA, Shimono Y, Qian D, Clarke MF. The biology of cancer stem cells. Annu Rev Cell Dev Biol. 2007; 23:675–699.
Article10. Lohberger B, Rinner B, Stuendl N, Absenger M, Liegl-Atzwanger B, Walzer SM, Windhager R, Leithner A. Aldehyde dehydrogenase 1, a potential marker for cancer stem cells in human sarcoma. PLoS One. 2012; 7:e43664.
Article11. MacEwen EG. Spontaneous tumors in dogs and cats: models for the study of cancer biology and treatment. Cancer Metastasis Rev. 1990; 9:125–136.
Article12. MacEwen EG, Hayes AA, Matus RE, Kurzman I. Evaluation of some prognostic factors for advanced multicentric lymphosarcoma in the dog: 147 cases (1978-1981). J Am Vet Med Assoc. 1987; 190:564–568.13. Montanaro F, Liadaki K, Schienda J, Flint A, Gussoni E, Kunkel LM. Demystifying SP cell purification: viability, yield, and phenotype are defined by isolation parameters. Exp Cell Res. 2004; 298:144–154.
Article14. Morton LM, Wang SS, Devesa SS, Hartge P, Weisenburger DD, Linet MS. Lymphoma incidence patterns by WHO subtype in the United States, 1992-2001. Blood. 2006; 107:265–276.
Article15. Patrawala L, Calhoun T, Schneider-Broussard R, Zhou J, Claypool K, Tang DG. Side population is enriched in tumorigenic, stem-like cancer cells, whereas ABCG2+ and ABCG2- cancer cells are similarly tumorigenic. Cancer Res. 2005; 65:6207–6219.
Article16. Ponce F, Magnol JP, Ledieu D, Marchal T, Turinelli V, Chalvet-Monfray K, Fournel-Fleury C. Prognostic significance of morphological subtypes in canine malignant lymphomas during chemotherapy. Vet J. 2004; 167:158–166.
Article17. Rabindran SK, Ross DD, Doyle LA, Yang W, Greenberger LM. Fumitremorgin C reverses multidrug resistance in cells transfected with the breast cancer resistance protein. Cancer Res. 2000; 60:47–50.18. Reya T, Morrison SJ, Clarke MF, Weissman IL. Stem cells, cancer, and cancer stem cells. Nature. 2001; 414:105–111.
Article19. Suzuyama N, Katoh M, Takeuchi T, Yoshitomi S, Higuchi T, Asashi S, Yokoi T. Species differences of inhibitory effects on P-glycoprotein-mediated drug transport. J Pharm Sci. 2007; 96:1609–1618.
Article20. Szotek PP, Pieretti-Vanmarcke R, Masiakos PT, Dinulescu DM, Connolly D, Foster R, Dombkowski D, Preffer F, Maclaughlin DT, Donahoe PK. Ovarian cancer side population defines cells with stem cell-like characteristics and Mullerian inhibiting substance responsiveness. Proc Natl Acad Sci U S A. 2006; 103:11154–11159.
Article21. Tirino V, Desiderio V, Paino F, De Rosa A, Papaccio F, La Noce M, Laino L, De Francesco F, Papaccio G. Cancer stem cells in solid tumors: an overview and new approaches for their isolation and characterization. FASEB J. 2013; 27:13–24.
Article22. Vail DM, MacEwen EG. Spontaneously occurring tumors of companion animals as models for human cancer. Cancer Invest. 2000; 18:781–792.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Isolation of Putative Limbal Epithelial Stem Cells by Fluorescein Activated Cell Sorting
- Effects of Glucocorticoid on Apoptosis of Human Bone Marrow Osteogenic Stromal Cells
- Isolation of Putative Corneal Epithelial Stem Cells from Cultured Limbal Tissue
- Anticancer effect of metformin alone and in combination with 2-deoxy-D-glucose on mouse T cell lymphoma EL4 cells
- Assessment of the permanent canine bone support after secondary bone graft in UCLP patients