Korean J Ophthalmol.
2006 Mar;20(1):55-61. 10.3341/kjo.2006.20.1.55.
Isolation of Putative Corneal Epithelial Stem Cells from Cultured Limbal Tissue
- Affiliations
-
- 1Department of Ophthalmology, Seoul National University Hospital, Seoul, Korea. wrwee@snu.ac.kr
- 2Seoul Artificial Eye Center, Seoul National University Hospital Clinical Research Institute, Seoul, Korea.
- 3Valued Eye Clinic, Taejon, Korea.
- 4Laboratory of Tissue Engineering, Korea Cancer Center Hospital, Seoul, Korea.
- KMID: 1099073
- DOI: http://doi.org/10.3341/kjo.2006.20.1.55
Abstract
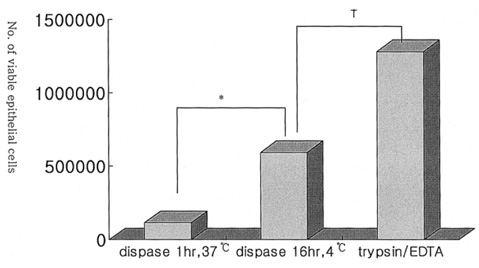
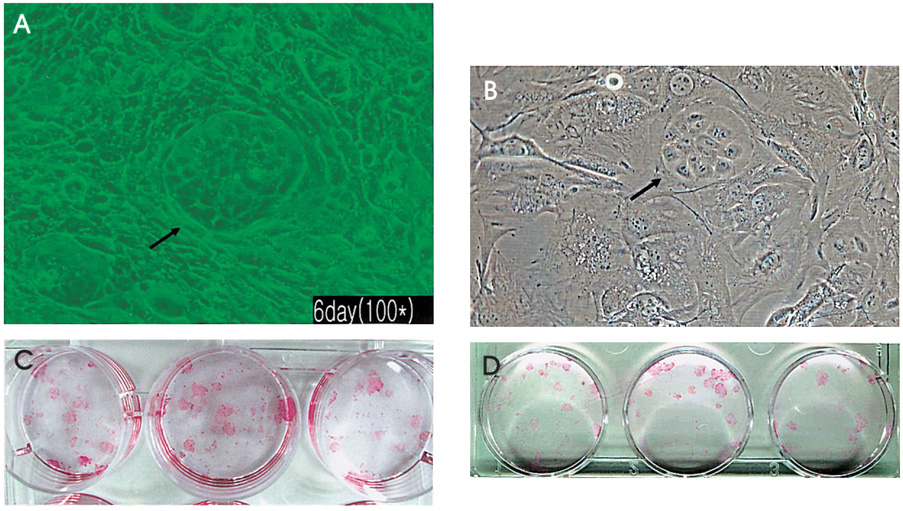
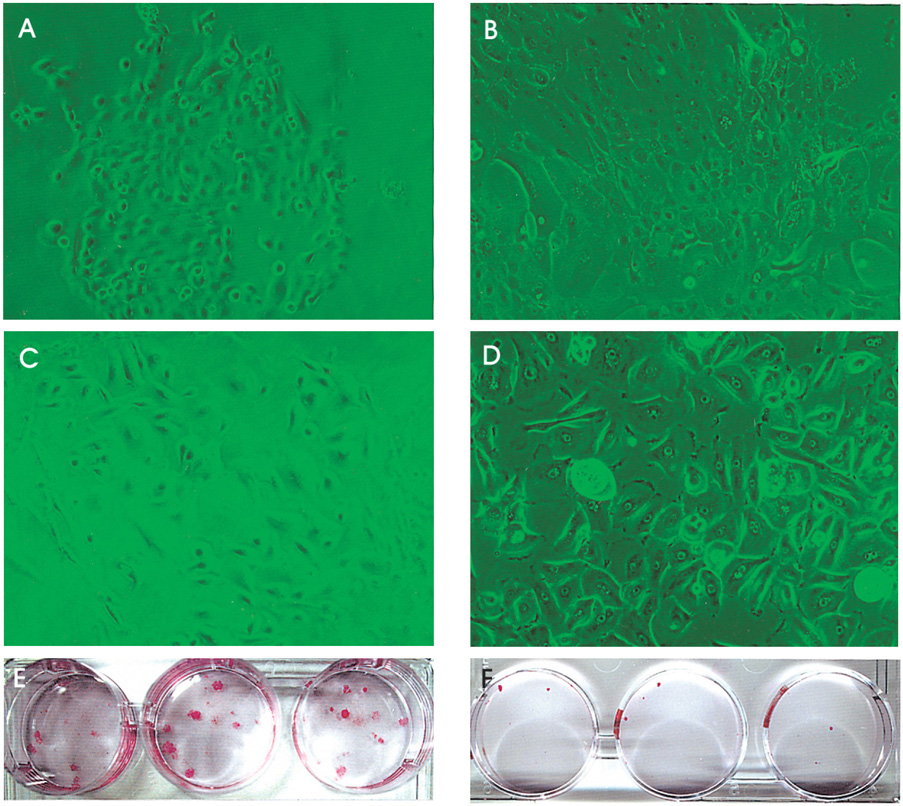
- PURPOSE: To investigate methods of isolating putative corneal epithelial stem cells from cultured limbal tissue. METHODS: Three extraction techniques were compared to identify an efficient method of obtaining a large number of viable corneal epithelial stem cells from the limbus. Limbal tissues were extracted by incubation at 37 degrees C or 4 degrees C for 1 or 16 hours, respectively, with 1.2U/ml dispase/trypsin or by treatment with 0.05% trypsin and 0.01% ethyldiaminetetraacetic acid (EDTA) at 37 degrees C in single procedure. Collected cells were cultured on NIH/3T3-seeded plates, and colony forming efficiency (CFE) was evaluated. Fluorescence activated cell sorting (FACS) was performed with a Coulter EPICS 753 after incubation with Hoechst 33342 and propidium iodide (PI). Hoechst negative cells were obtained using gates exhibiting low Hoechst blue with a 424/44 nm BP filter. Gated cells of each fraction were re-cultured to assess the capability of colony formation. RESULTS: The mean numbers of viable cells obtained from treatment with dispase and trypsin was 3x10(4) cell/ml and 8.06x10(5) cell/ml at 37 degrees C and 4 degrees C incubations; the number increased to 1.21x10(6) cell/ml with a trypsin/EDTA treatment (p<0.05). CFE was 9.67+/-2.13% and 6.63+/-2.35% in rabbit and human cells, respectively. Likewise, the Hoechst negative fraction was 3.61+/-0.42% and 5.21+/-4.91% in rabbit and human cells, respectively. The sorted Hoechst negative cells were cultured through four passages, forming small round colonies. In rabbit cells, the CFEs of Hoechst negative and positive fractions after FACS, were 12.67+/-2.24% and 1.17+/-6.13%, respectively (p<0.05). CONCLUSIONS: Putative corneal epithelial stem cells were efficiently isolated from limbal tissue using a trypsin/EDTA extraction and FACS. This technique may be very useful in tissue engineered stem cell therapy.
Keyword
MeSH Terms
Figure
Cited by 1 articles
-
The Effects of Wnt Protein on Proliferation and Stemness Maintenance of Corneal Limbal Stem Cells (CLSCs)
Hyun-Jin Jin, Choun-Ki Joo
J Korean Ophthalmol Soc. 2009;50(4):588-593. doi: 10.3341/jkos.2009.50.4.588.
Reference
-
1. Tsai RJ, Tseng SC. Human allograft limbal transplantation for corneal surface reconstruction. Cornea. 1994. 13:389–400.2. Tsubota K, Satake Y, Ohyama M, et al. Surgical reconstruction of the ocular surface in advanced ocular cicatricial pemphigoid and Stevens-Johnson syndrome. Am J Ophthalmol. 1996. 122:38–52.3. Pellegrini G, Traverso CE, Franzi AT, et al. Long-term restoration of damaged corneal surfaces with autologous cultivated corneal epithelium. Lancet. 1997. 349:990–993.4. Tsai RJ, Li LM, Chen JK. Reconstruction of damaged corneas by transplantation of autologous limbal epithelial cells. N Engl J Med. 2000. 343:86–93.5. Lindberg K, Brown ME, Chaves HV, et al. In vitro propagation of human ocular surface epithelial cells for transplantation. Invest Ophthalmol Vis Sci. 1993. 34:2672–2679.6. Tseng SC, Kruse FE, Merritt J, et al. Comparison between serum-free and fibroblast-cocultured single-cell clonal culture systems: evidence showing that epithelial antiapoptotic activity is present in 3T3 fibroblast-conditioned media. Curr Eye Res. 1996. 15:973–984.7. Davanger M, Evensen A. Role of the pericorneal papillary structure in renewal of corneal epithelium. Nature. 1971. 229:560–561.8. Cotsarelis G, Cheng SZ, Dong G, et al. Existence of slow-cycling limbal epithelial basal cells that can be preferentially stimulated to proliferate: implications on epithelial stem cells. Cell. 1989. 57:201–209.9. Huang AJ, Tseng SC. Corneal epithelial wound healing in the absence of limbal epithelium. Invest Ophthalmol Vis Sci. 1991. 32:96–105.10. Schermer A, Galvin S, Sun TT. Differentiation-related expression of a major 64K corneal keratin in vivo and in culture suggests limbal location of corneal epithelial stem cells. J Cell Biol. 1986. 103:49–62.11. Ploemacher RE, Brons NH. The relative spatial distribution of CFU-S in the mouse spleen. Exp Hematol. 1985. 13:1068–1072.12. Pallavicini MG, Summers LJ, Dean PN, et al. Enrichment of murine hemopoietic clonogenic cells by multivariate analyses and sorting. Exp Hematol. 1985. 13:1173–1181.13. Spangrude GJ, Johnson GR. Resting and activated subsets of mouse multipotent hematopoietic stem cells. Proc Natl Acad Sci U S A. 1990. 87:7433–7437.14. Scharenberg CW, Harkey MA, Torok-Storb B. The ABCG2 transporter is an efficient Hoechst 33342 efflux pump and is preferentially expressed by immature human hematopoietic progenitors. Blood. 2002. 99:507–512.15. Goodell MA, Rosenzweig M, Kim H, et al. Dye efflux studies suggest that hematopoietic stem cells expressing low or undetectable levels of CD34 antigen exist in multiple species. Nat Med. 1997. 3:1337–1345.16. McAlister I, Wolf NS, Pietrzyk ME, et al. Transplantation of hematopoietic stem cells obtained by a combined dye method fractionation of murine bone marrow. Blood. 1990. 75:1240–1246.17. Wolf NS, Kone A, Priestley GV, et al. In vivo and in vitro characterization of long-term repopulating primitive hematopoietic cells isolated by sequential Hoechst 33342-rhodamine 123 FACS selection. Exp Hematol. 1993. 21:614–622.18. Jackson KA, Mi T, Goodell MA. Hematopoietic potential of stem cells isolated from murine skeletal muscle. Proc Natl Acad Sci U S A. 1999. 96:14482–14486.19. Gussoni E, Soneoka Y, Strickland CD, et al. Dystrophin expression in the mdx mouse restored by stem cell transplantation. Nature. 1999. 401:390–394.20. Dunnwald M, Tomanek-Chalkley A, Alexandrunas D, et al. Isolating a pure population of epidermal stem cells for use in tissue engineering. Exp Dermatol. 2001. 10:45–54.21. de Paiva CS, Chen Z, Corrales RM, et al. ABCG2 transporter identifies a population of clonogenic human limbal epithelial cells. Stem Cells. 2005. 23:63–73.22. Watanabe K, Nishida K, Yamato M, et al. Human limbal epithelium contains side population cells expr essing the ATP-binding cassette transporter ABCG2. FEBS Lett. 2004. 565:6–10.23. Gipson IK, Grill SM. A technique for obtaining sheets of intact rabbit corneal epithelium. Invest Ophthalmol Vis Sci. 1982. 23:269–273.24. Trinkaus-Randall V, Gipson IK. A technique for obtaining basal corneal epithelial cells. Invest Ophthalmol Vis Sci. 1985. 26:233–237.25. Kruse FE, Tseng SC. A serum-free clonal growth assay for limbal, peripheral, and central corneal epithelium. Invest Ophthalmol Vis Sci. 1991. 32:2086–2095.26. Koizumi N, Cooper LJ, Fullwood NJ, et al. An evaluation of cultivated corneal limbal epithelial cells, using cell-suspension culture. Invest Ophthalmol Vis Sci. 2002. 43:2114–2121.27. Espana EM, Romano AC, Kawakita T, et al. Novel enzymatic isolation of an entire viable human limbal epithelial sheet. Invest Ophthalmol Vis Sci. 2003. 44:4275–4281.28. Pellegrini G, Golisano O, Paterna P, et al. Location and clonal analysis of stem cells and their differentiated progeny in the human ocular surface. J Cell Biol. 1999. 145:769–782.29. Murayama A, Matsuzaki Y, Kawaguchi A, et al. Flow cytometric analysis of neural stem cells in the developing and adult mouse brain. J Neurosci Res. 2002. 69:837–847.30. Budak MT, Alpdogan OS, Zhou M, et al. Ocular surface epithelia contain ABCG2-dependent side population cells exhibiting features associated with stem cells. J Cell Sci. 2005. 118:1715–1724.31. Machalinski B, Wiszniewska B, Baskiewicz M, et al. In vivo and in vitro studies on the toxicity of Hoechst 33342 (Ho342). Implications for employing Ho342 for the isolation of haematopoietic stem cells. Ann Transplant. 1998. 3:5–13.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Transplantation of in vivo Cultivated Limbal Corneal Epithelial Cells with Total Limbal Stem Cell Deficiency
- Long-term Outcome of Limbal Epithelial Cells Cultivated in Vivo on Amniotic Membrane Transplantation
- Peripheral Blood As a Source of Stem Cells for RegenerativeMedicine: Emphasis Towards Corneal EpithelialReconstruction—An In Vitro Study
- Migration of Normal Corneal Epithelial Cells in Rats
- The Effect of A Polypeptide Factor Released by Polymorphonuclear Leukocytes (PMNs Factor)on Rabbit Corneal Epithelial Stem Cells