J Korean Soc Spine Surg.
2007 Mar;14(1):44-51. 10.4184/jkss.2007.14.1.44.
Spinal Cord Injury without Radiographic Abnormality in Adults
- Affiliations
-
- 1Department of Orhtopaedic Surgery, Yonsei University, Wonju College of Medicine, Wonju, Korea. par73@yonsei.ac.kr
- 2Department of Radiology, Yonsei University, Wonju College of Medicine, Wonju, Korea.
- KMID: 1527142
- DOI: http://doi.org/10.4184/jkss.2007.14.1.44
Abstract
-
STUDY DESIGN: This is a retrospective study.
OBJECTIVES
This study examined the MRI findings, injury mechanism, clinical findings, and prognosis of a spinal cord injury without radiographic abnormality (SCIWORA) in adults with a normal spinal canal. SUMMARY OF LITERTURE: Most reports on SCIWORA deal with the pediatric age group. However, there are few reports on the MRI findings, clinical features and outcomes in adult patients with cervical SCIWORA.
MATERIALS AND METHODS
The hospital records of 753 patients, who were treated for cervical spine injury between February 1, 1994 and July 31, 2004, were reviewed. This study included the 10 subjects with no fractures or dislocation on the plain roentgenograms or cord compression caused by degenerative change or disc herniation on MRI corresponding to the location of the cord lesion. All the patients had at least a 2-year follow-up evaluation. The relationships between the MRI findings, neurological findings and outcomes were evaluated.
RESULTS
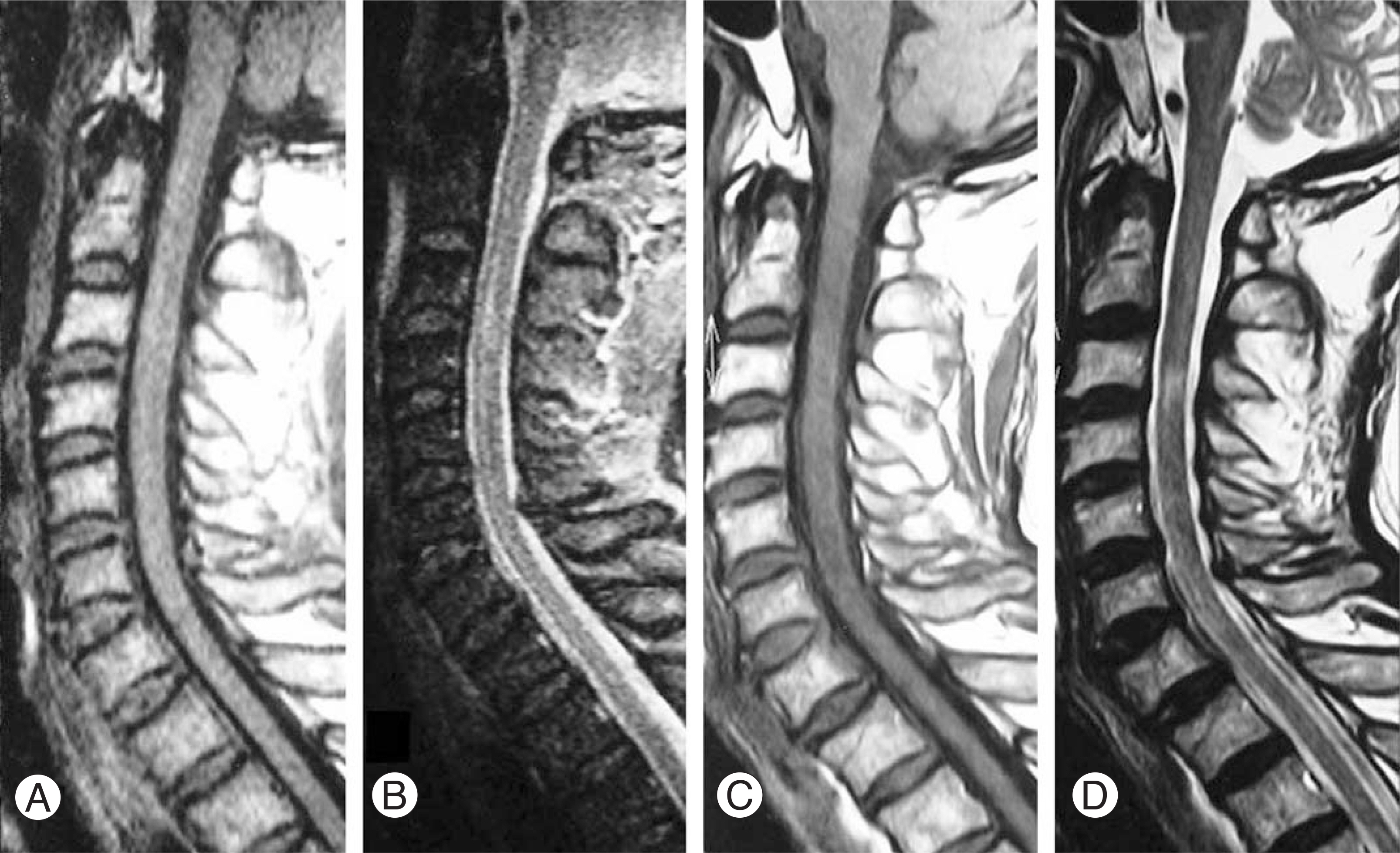
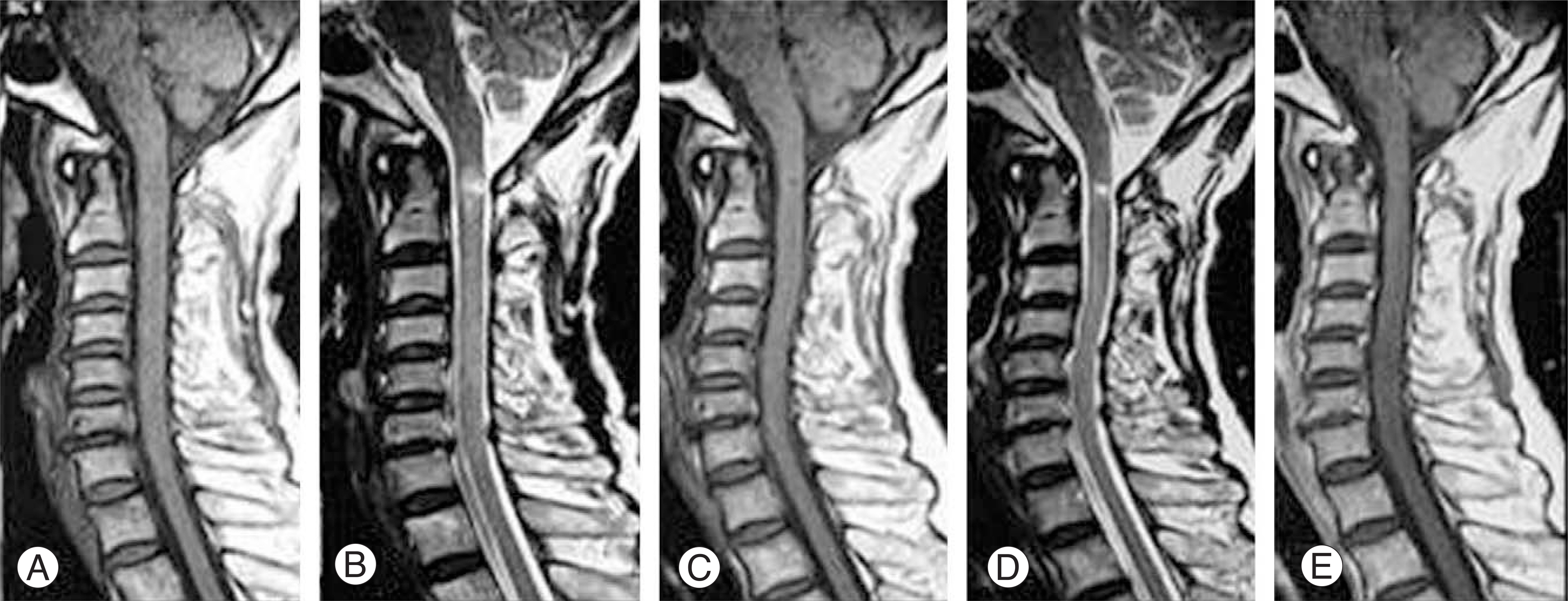
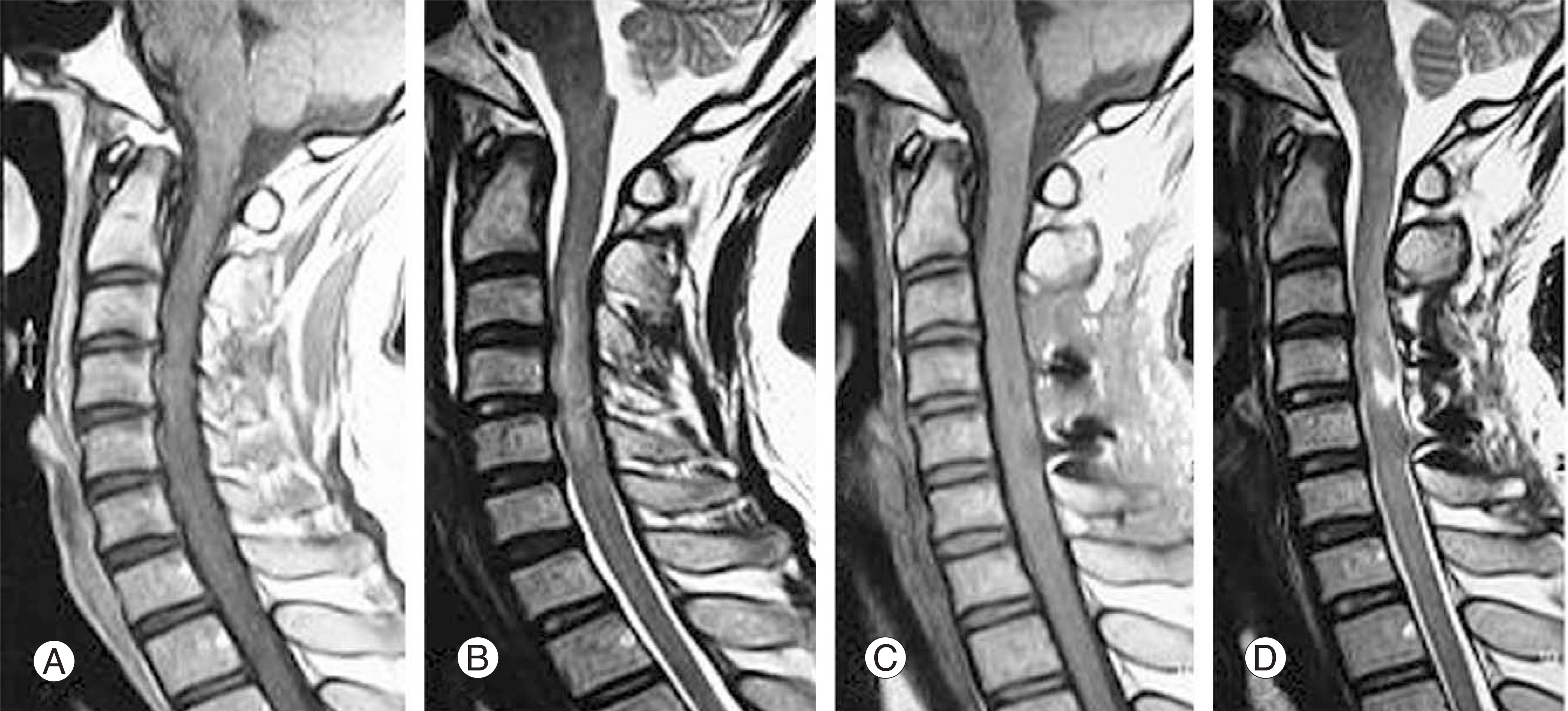
The MRI findings revealed 7 cases with cord contusion, 3 cases with cord edema, 3 cases with gliosis and 3 cases with syrinx formation at the follow-up. The injury mechanism was hyperextension and hyperflexion in 7 and 3 cases, respectively. The initial motor function scores of ASIA in the edema and contusion groups was 60.7 and 43.9, respectively. At the last follow-up, the motor function scores of ASIA in the edema and contusion groups were 90 and 70.3, respectively. The Frankel grade improved by 1.3 and 1.1 in the edema and contusion groups, respectively.
CONCLUSIONS
In patients with SCIWORA, the MRI findings correlated well with the clinical picture and were of prognostic significance. The cord edema group showed better clinical features than the contusion group, and prognosis was relatively good in both groups. A further careful evaluation, such as MRI, is still needed to determine the appropriate treatment for spinal cord injuries without radiographic abnormalities.
Keyword
MeSH Terms
Figure
Reference
-
1). Pang D, Wilberger JE Jr. Spinal cord injury without radiographic abnormalities in children. J Neurosurg. 1982; 57:114–129.
Article2). Choi JU, Hoffman HJ, Hendrick EB, Humphreys RP, Keith WS. Traumatic infarction of the spinal cord in children. J Neurosurg. 1986; 65:608–610.
Article3). Hill SA, Miller CA, Kosnik EJ, Hunt WE. Pediatric neck injuries: A clinical study. J Neurosurg. 1984; 60:700–706.4). Ruge JR, Sinson GP, McLone DG, Cerullo LJ. Pediatric spinal injury: The very young. J Neurosurg. 1988; 68:25–30.
Article5). Firooznia H, Ahn JH, Rafii M, Ragnarsson KT. Sudden quadriplegia after a minor trauma. The role of preexisting spinal stenosis. Surg Neurol. 1985; 23:165–168.
Article6). Silberstein M, Hnnessy DO. Implications of focal spinal cord lesions following trauma: evaluation with magnetic resonance imaging. Paraplegia. 1993; 31:160–167.
Article7). Frankel HL, Hancock DO, Hyslop G, et al. The value of postural reduction in the initial management of closed injuries of the spine with paraplegia and tetraplegia. I. Paraplegia. 1969; 7:179–192.8). Gupta SK, Rajeev K, Khosla VK, et al. Spinal cord injury without radiographic abnormality in adults. Spinal cord. 1999; 37:726–729.
Article9). Chirossel JP, Vanneuville G, Passagia JG, et al. Biome-chanics and classification of traumatic lesions of the spine. Adv Tech Stand Neurosurg. 1995; 22:55–135.
Article10). Lyness SS, Wagman AD. Neurological deficits following cervical manipulation. Surg Neurol. 1974; 2:121–124.11). Wilder BL. Hypothesis: the etiology of midcervical quadriplegia after operation with the patient in the sitting position. Neurosurg. 1982; 11:530–531.
Article12). Taylor AR. The mechanism of injury to the spinal cord in the neck without damage to the vertebral column. J Bone Joint Surg Br. 1951; 33B:543–547.
Article13). Kobrine AI. The neuronal theory of experimental traumatic spinal cord dysfunction. Surg Neurol. 1975; 3:261–264.14). Simpson RK Jr, Robertson DP, Narayan RK. Penetrating spinal cord injury. (in. Narayan RK, Wilberger JE, Povlishock JT, editors. Neurotrauma. New York: McGraw-Hill Inc.;p. 1289–1300. 1996. .).15). Holmes G. Spinal injuries of warfare. Br Med J. 1915; 2:769–774. (cited from Gupta SK, Rajeev K, Khosla VK et al: Spinal cord injury without radiographic abnormality in adults. Spinal cord. 1999; 37:726–729. .).16). Bhatoe HS. Cervical spinal cord injury without radiological abnormality in adults. Neurol India. 2000; 48:243–248.17). Bondurant FJ, Cotler HB, Kulkarni MV, McArdle CB, Harris JH Jr. Acute spinal cord injury, A study using physical examination and magnetic resonance imaging. Spine. 1990; 15:161–168.18). Flanders AE, Schaefer DM, Doan HT, Mishkin MM, Gonzalez CF, Northrup BE. Acute cervical spine trauma: correlation of MR imaging findings with degree of neurological deficit. Radiology. 1990; 177:25–33.19). Marciello MA, Flanders AE, Herbison GJ, Schaefer DM, Friedman DP, Lane JI. Magnetic resonance imaging related to neurologic outcome in cervical spinal cord injury. Arch Phys Med Rehabil. 1993; 74:940–946.20). Sagiuchi T, Tachibana S, Endo M, Hayakawa K. Diffusion-weighted MRI of the cervical cord in acute spinal cord injury with type II odontoid fracture. J Comp Assis Tomogr. 2002; 26:654–656.
Article21). Tsuchiya K, Katase S, Fujikawa A, Hachiya J, Kanaza-wa H, Yodo K. Diffusion weighted MRI of the cervical spinal cord using a single-shot fast spin-echo technique: indings in normal subjects and in myelomalacia. Nerora-diololgy. 2003; 45:90–94.22). Collins WF. A review and update of experiment and clinical studies of spinal cord injury. Paraplegia. 1983; 21:204–219.
Article23). Tator CH, Fehlings MG. Review of the secondary injury theory of acute spinal cord trauma with emphasis on vascular mechanisms. J Neurosurg. 1991; 75:15–26.
Article24). Senter HJ, Venes JL. Loss of autoregulation and post-traumatic ischemia following experimental spinal cord trauma. J Neurosurg. 1979; 50:198–206.
Article25). Geisler FH, Coleman WP, Grieco G, Poonian D. The Sygen multicenter acute spinal cord injury study. Spine. 2001; 26:S87–98.
Article26). Geisler FH, Dorsey FC, Coleman WP. Recovery of motor function after spinal-cord injury- a randomized, placebo-controlled trial with GM-1 ganglioside. N Engl J Med. 1991; 324:1829–1838.27). Eck JC, Nachtigall D, Humphreys C, Hodges SD. Questionnaire survey of spine surgeons on the use of methylprednisolone for acute spinal cord injury. Spine. 2006; 31:E250–253.
Article28). Sayer FT, Kronvall E, Nilsson OG. Methylprednisolone treatment in acute spinal cord injury: the myth challenged through a structured analysis of published literature. Spine J. 2006; 6:335–343.
Article29). Laud NS, Ramani PS. Patterns of spinal injuries. (in. Ramani PS, editor. Spinal Surgery Vol I. Dept of Neuro and Spinal Surgery, Mumbai: LTM Medical College;p. 185–192. 1996. .).
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Thoracic Spinal Cord Injury without Radiographic Abnormality Following Minor Trauma in Adult
- A Case of Traumatic Cardiac Arrest due to SCIWORA (Spinal Cord Injury without Radiographic Abnormality)
- Traumatic Atypical Tetraplegia Without Radiologic Abnormalities Including Magnetic Resonance Imaging in an Adult: A Case Report
- A Case of Spinal Cord Injury without Radiographic Abnormality
- The Role of MRI in Spinal Cord Injury Without Radiographic Abnormality