J Korean Endocr Soc.
2007 Oct;22(5):359-364. 10.3803/jkes.2007.22.5.359.
A Case of Ectopic ACTH Syndrome Associated with Small Cell Lung Cancer Presented with Hypokalemia
- Affiliations
-
- 1Department of Internal Medicine, College of Medicine, The Catholic University of Korea.
- KMID: 1523106
- DOI: http://doi.org/10.3803/jkes.2007.22.5.359
Abstract
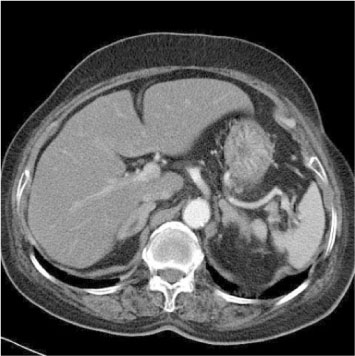
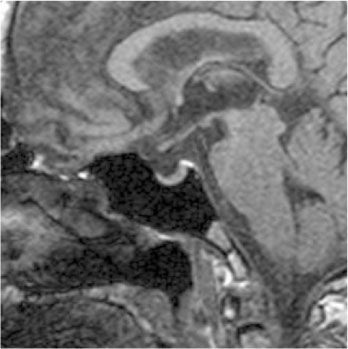
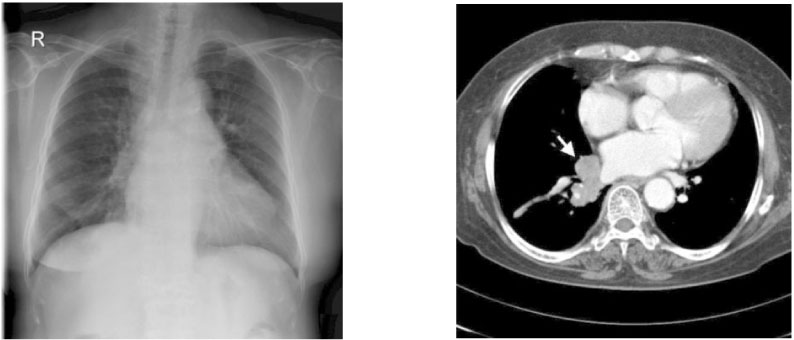
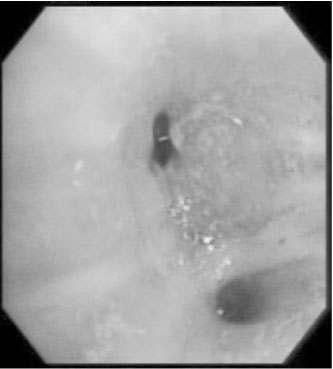
- We report a case of a 73-year-old female patient who was diagnosed with ectopic ACTH syndrome caused by small cell lung cancer. We initially presumed that the patient was in a state of mineralocorticoid excess, because she had hypertension and hypokalemic alkalosis. This was however excluded because her plasma renin activity was not suppressed and her plasma aldosterone/plasma renin activity ratio was below 25. Moreover, her 24 hour urine free cortisol level was elevated and her serum cortisol levels after a low dose dexamethasone suppression test, were not suppressed. Furthermore, her basal plasma ACTH and serum cortisol levels increased and her serum cortisol level after a high dose dexamethasone suppression test was not suppressed. We performed studies to identify the source of ectopic ACTH syndrome and found a 3 cm-sized mass in the patient's right lower lobe of her lung, which was eventually diagnosed as small cell lung cancer following a bronchoscopic biopsy. In conclusion, Cushing's syndrome, and in particular ectopic ACTH syndrome, must be considered in the differential diagnosis of mineralocorticoid-induced hypertension. The excessive cortisol saturates the 11beta-hydroxysteroid dehydrogenase type 2 (11beta-HSD2) activity, which in turn, inactivates the conversion of cortisol to cortisone in the renal tubules. Moreover, excessive cortisol causes binding to the mineralocorticoid receptors, causing mineralocorticoid hypertension, characterized by severe hypercortisolism.
MeSH Terms
-
11-beta-Hydroxysteroid Dehydrogenase Type 2
ACTH Syndrome, Ectopic*
Adrenocorticotropic Hormone
Aged
Alkalosis
Biopsy
Cortisone
Cushing Syndrome
Dexamethasone
Diagnosis, Differential
Female
Humans
Hydrocortisone
Hypertension
Hypokalemia*
Lung
Patient Rights
Plasma
Receptors, Mineralocorticoid
Renin
Small Cell Lung Carcinoma*
11-beta-Hydroxysteroid Dehydrogenase Type 2
Adrenocorticotropic Hormone
Cortisone
Dexamethasone
Hydrocortisone
Receptors, Mineralocorticoid
Renin
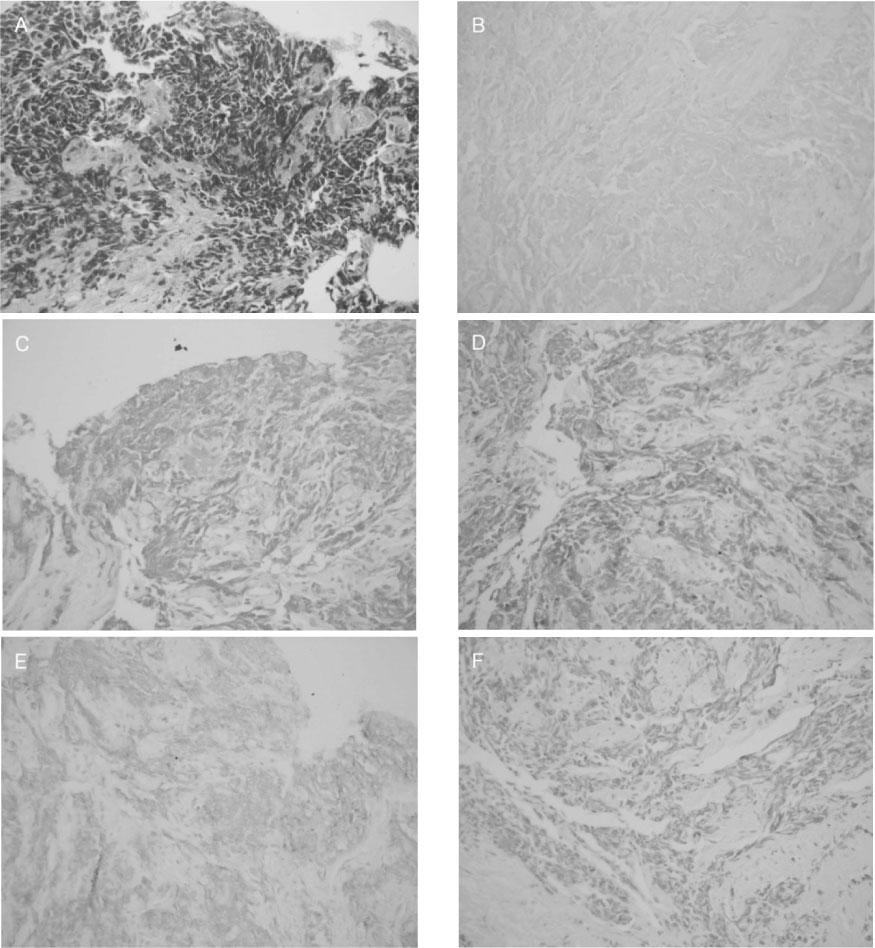
Figure
Reference
-
1. Stewart PM. Mineralocorticoid hypertension. Lancet. 1999. 353:1341–1347.2. Liddle GW, Island DP, Ney RL, Nicholson WE, Shimizu N. Nonpituitary neoplasms and Cushing's syndrome. Ectopic "adrenocorticotropin" produced by nonpituitary neoplasms as a cause of Cushing's syndrome. Arch Intern Med. 1963. 111:471–475.4. Kim SK, Hong KI, Lee YC, Lee SH, Nam SH, Park JS, Park SW. A case of ectopic ACTH syndrome associated with small cell carcinoma of the lung. 1987. 11:548–553.5. Nieman LK, Ilias I. Evaluation and treatment of Cushing's syndrome. Am J Med. 2005. 118:1340–1346.6. Orth DN. Cushing's syndrome. N Engl J Med. 1995. 332:791–803.7. Larsen PE. Williams Textbook of Endocrinology. 2006. 10th ed. Elsevier;1842–1844.8. Ilias I, Torpy DJ, Pacak K, Mullen N, Wesley RA, Nieman LK. Cushing's syndrome due to ectopic corticotropin secretion: Twenty years' experience at the National Institutes of Health. J Clin Endocrinol Metab. 2005. 90:4955–4962.9. Kasper DL, Braunwald E, Fauci AS, Hauser SL, Longo DL, Jameson JL. Harrison's Principles of Internal Medicine. 2004. 16th ed. McGraw-Hill;258–261.10. Quinkler M, Stewart PM. Hypertension and the cortisol-cortisone shuttle. J Clin Endocrinol Metab. 2003. 88:2384–2392.11. Edwards CR, Stewart PM, Burt D, Brett L, McIntyre MA, Sutanto WS, de Kloet ER, Monder C. Localisation of 11β-hydroxysteroid dehydrogenase-tissue specific protector of the mineralocorticoid receptor. Lancet. 1988. 2:986–989.12. Funder JW, Pearce PT, Smith R, Smith AI. Mineralocorticoid action: target tissue specificity is enzyme, not receptor, mediated. Science. 1988. 242:583–585.13. Isidori AM, Kaltsas GA, Pozza C, Frajese V, Newell-Price J, Reznek RH, Jenkins PJ, Monson JP, Grossman AB, Besser GM. The ectopic adrenocorticotropin syndrome: clinical features, diagnosis, management, and long-term follow-up. J Clin Endocrinol Metab. 2006. 91:371–377.14. Wajcheonberg BL, Mendonca BB, Liberman B, Pereira MA, Carneiro PC, Wakamatsu A, Kirschner MA. Ectopic adrenocorticotropic hormone syndrome. Endocr Rev. 1994. 15:752–787.15. Heitz PU, Kloppel G, Polak JM, Staub JJ. Ectopic hormone production by endocrine tumors: localization of hormones at the cellular level by immunocytochemistry. Cancer. 1981. 48:2029–2037.16. Sarlis NJ, Chanock SJ, Nieman LK. Cortisolemic indices predict severe infections in Cushing syndrome due to ectopic production of adrenocorticotropin. J Clin Endocrinol Metab. 2000. 85:42–47.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- A Case of Cushing's Syndrome Associated with Ectopic ACTH Production in Patient with Small: cell Lung Cancer
- A Case of Ectopic Adrenocorticotropic Hormone Syndrome in Small Cell Lung Cancer
- A Case of Ectopic ACTH Syndrome Associated with Small Cell Lung Cancer
- A Case of Ectopic ACTH Syndrome Associated with Metastatic Prostate Cancer
- A Case of Ectopic ACTH Syndrome Associated with Bronchial Carcinoid