J Bacteriol Virol.
2007 Mar;37(1):31-38. 10.4167/jbv.2007.37.1.31.
Production of Recombinant Retroviruses Encoding Human Flt3 Ligand and IL-6 and Establishment of Genetically Modified OP9 Mouse Stromal Cells
- Affiliations
-
- 1Department of Biochemistry, College of Natural Sciences, Chungbuk National University, Cheongju, Chungbuk, 361-763, Korea. yhl4177@chungbuk.ac.kr
- 2Department of Pharmacy, College of Pharmacy, Chungnam National University, Yusong, Daejon, 305-764, Korea.
- KMID: 1513550
- DOI: http://doi.org/10.4167/jbv.2007.37.1.31
Abstract
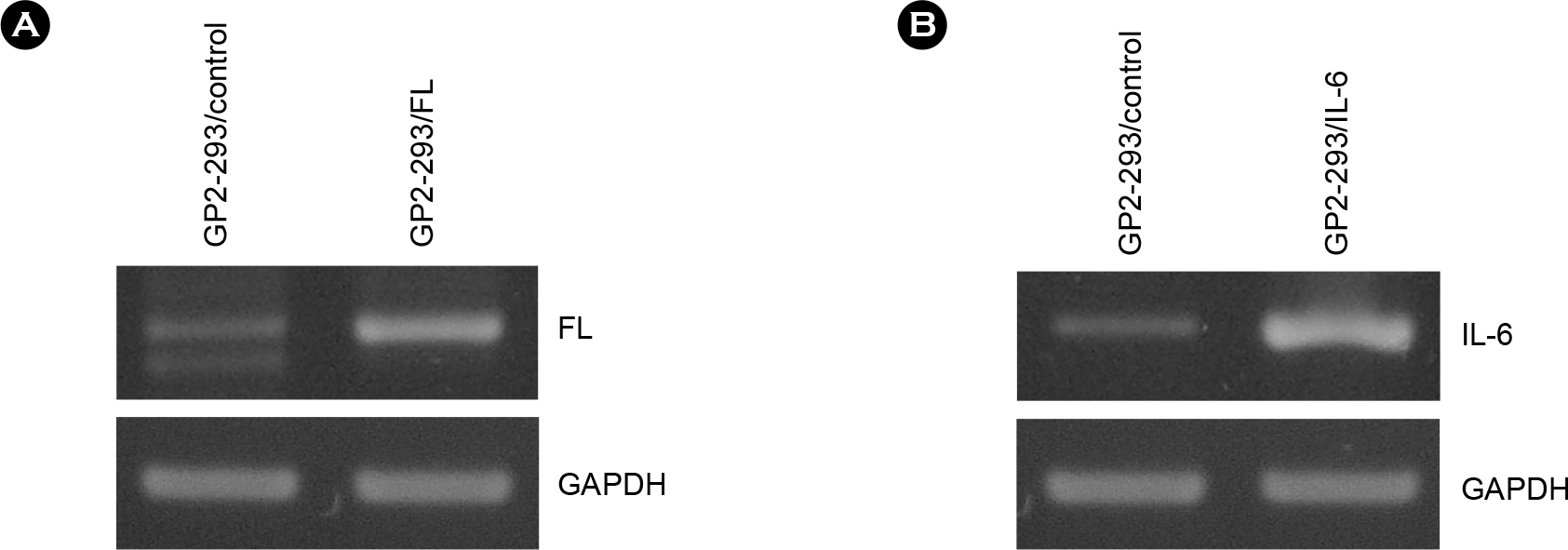
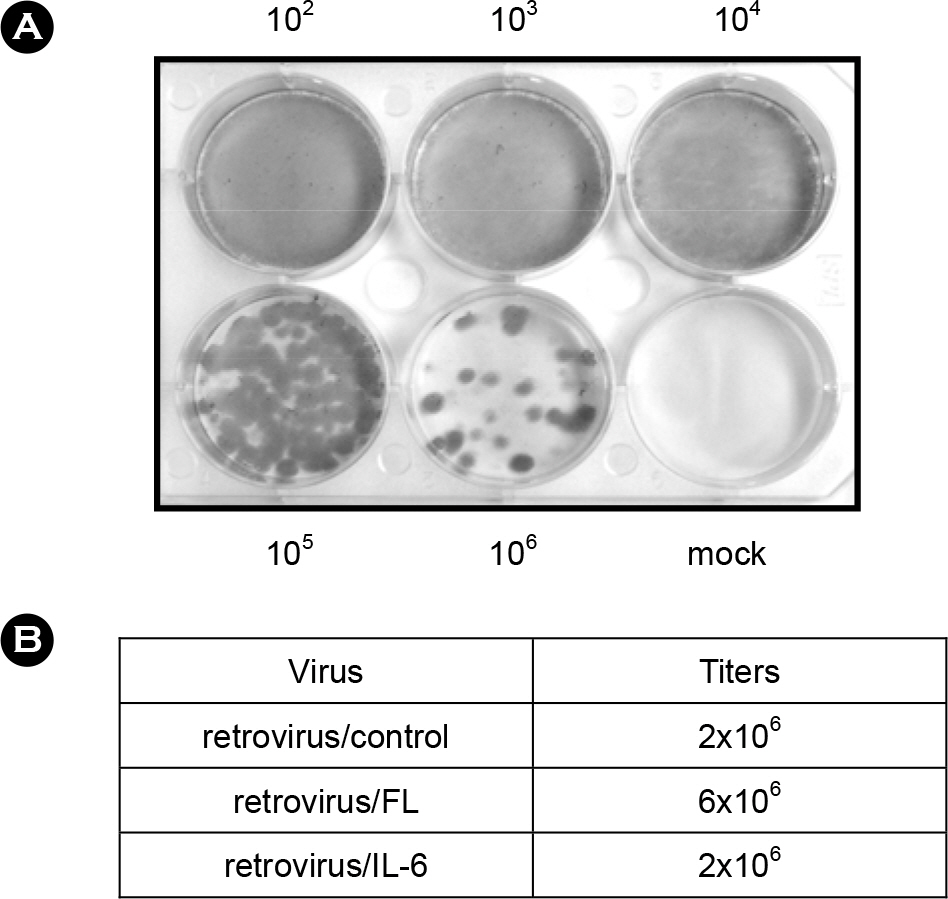
- Flt3 Ligand (FL) and IL-6 are multifunctional cytokines implicated in normal hematopoiesis and ex vivo expansion of hematopoietic stem cells. Retroviral vectors are useful for stable expression of genes in many cells. Here, we aimed to produce retroviral vectors directing expression of human FL and IL-6 genes. Recombinant retroviral vectors containing human genes for FL and IL-6 were constructed using a retroviral vector pLXSN. Recombinant retroviruses were produced from GP2-293 cells with the aid of pseudo-envelope protein gene VSV-G, and efficiently transduced to a mouse stromal cell line OP9. Genetically modified OP9 cells clearly showed expression of human FL or IL-6 gene at the mRNA level determined by RT-PCR. Based on the results from ELISA, human FL and IL-6 were detected in the cell culture medium of OP9/FL and OP9/IL-6 cells, respectively. As the recombinant human FL and IL-6 proteins are successfully produced and secreted to the culture medium, this system can be useful in future application such as ex vivo expansion of hematopoietic stem cells and differentiation of embryonic stem cells.
Keyword
MeSH Terms
Figure
Reference
-
References
1). Amit M, Carpenter MK, Inokuma MS, Chiu CP, Harris CP, Waknitz MA, Itskovitz-Eldor J, Thomson JA. Clonally derived human embryonic stem cell lines maintain pluripotency and proliferative potential for prolonged periods of culture. Dev Biol. 227:271–278. 2000.
Article2). Bauer J, Herrmann F. Interleukin-6 in clinical medicine. Ann Hematol. 62:203–210. 1991.
Article3). Bhatia M, Bonnet D, Wu D, Murdoch B, Wrana J, Gallacher L, Dick JE. Bone morphogenetic proteins regulate the developmental program of human hematopoietic stem cells. J Exp Med. 189:1139–1148. 1999.
Article4). Broxmeyer HE. Biology of cord blood cells and future prospects for enhanced clinical benefit. Cytotherapy. 7:209–218. 2005.
Article5). Broxmeyer HE. The hematopoietic system: principles of therapy with hematopoietically active cytokines. pp. p. 1–37. In. Cytokines in the treatment of hematopoietic failure. Ganser A, Hoelzer D, editors. (Ed),. Marcel Dekker;New York: 1999.6). Broxmeyer HE. Myelosuppressive cytokines and peptides. pp. p. 121–150. In. Blood cell biochemistry, Vol 7. Hemopoietic growth factors. Whetton T, Gordon T, editors. (Ed),. Kluwer Academic/Plenum Publishers;London, UK: 1996.
Article7). Chi L, Li Y, Stehno-Bittel L, Gao J, Morrison DC, Stechschulte DJ, Dileepan KN. Interleukin-6 production by endothelial cells via stimulation of protease-activated receptors is amplified by endotoxin and tumor necrosis factor-alpha. J Interferon Cytokine Res. 21:231–240. 2001.8). Chu P, Lutzko C, Stewart AK, Dube ID. Retrovirus-mediated gene transfer into human hematopoietic stem cells. J Mol Med. 76:184–192. 1998.
Article9). Dorshkind K. Regulation of hemopoiesis by bone marrow stromal cells and their products. Annu Rev Immunol. 8:111–137. 1990.
Article10). Eto K, Murphy R, Kerrigan SW, Bertoni A, Stuhlmann H, Nakano T, Leavitt AD, Shattil SJ. Megakaryocytes derived from embryonic stem cells implicate CalDAG-GEFI in integrin signaling. Proc Natl Acad Sci U S A. 99:12819–12824. 2002.
Article11). Hammacher A, Ward LD, Weinstock J, Treutlein H, Yasukawa K, Simpson RJ. Structure-function analysis of human IL-6: identification of two distinct regions that are important for receptor binding. Protein Sci. 3:2280–2293. 1994.
Article12). Hannum C, Culpepper J, Campbell D, McClanahan T, Zurawski S, Bazan JF, Kastelein R, Hudak S, Wagner J, Mattson J, et al. Ligand for FLT3/FLK2 receptor tyrosine kinase regulates growth of haematopoietic stem cells and is encoded by variant RNAs. Nature. 368:643–648. 1994.
Article13). Hirano T. The biology of interleukin-6. Chem Immunol. 51:153–180. 1992.14). Itskovitz-Eldor J, Schuldiner M, Karsenti D, Eden A, Yanuka O, Amit M, Soreq H, Benvenisty N. Differentiation of human embryonic stem cells into embryoid bodies compromising the three embryonic germ layers. Molecular Medicine (Cambridge, Mass.). 6:88–95. 2000.15). Jacobsen SE, Okkenhaug C, Myklebust J, Veiby OP, Lyman SD. The FLT3 ligand potently and directly stimulates the growth and expansion of primitive murine bone marrow progenitor cells in vitro: synergistic interactions with interleukin (IL) 11, IL-12, and other hematopoietic growth factors. J Exp Med. 181:1357–1363. 1995.
Article16). Kee BL, Paige CJ. Murine B cell development: commitment and progression from multipotential progenitors to mature B lymphocytes. Int Rev Cytol. 157:129–179. 1995.
Article17). Kincade PW, Lee G, Pietrangeli CE, Hayashi S, Gimble JM. Cells and molecules that regulate B lymphopoiesis in bone marrow. Annu Rev Immunol. 7:111–143. 1989.
Article18). Kishimoto T, Akira S, Narazaki M, Taga T. Interleukin 6 family of cytokines and gp130. Blood. 86:1243–1254. 1995.19). Ku H, Yonemura Y, Kaushansky K, Ogawa M. Thrombopoietin, the ligand for the Mpl receptor, synergizes with steel factor and other early acting cytokines in supporting proliferation of primitive hematopoietic progenitors of mice. Blood. 87:4544–4551. 1996.
Article20). Kurian KM, Watson CJ, Wyllie AH. Retroviral vectors. Mol Pathol. 53:173–176. 2000.
Article21). Le JM, Vilcek J. Interleukin 6: a multifunctional cytokine regulating immune reactions and the acute phase protein response. Lab Invest. 61:588–602. 1989.
Article22). McKenna HJ, Stocking KL, Miller RE, Brasel K, De Smedt T, Maraskovsky E, Maliszewski CR, Lynch DH, Smith J, Pulendran B, Roux ER, Teepe M, Lyman SD, Peschon JJ. Mice lacking flt3 ligand have deficient hematopoiesis affecting hematopoietic progenitor cells, dendritic cells, and natural killer cells. Blood. 95:3489–3497. 2000.
Article23). Nagasawa T. A chemokine, SDF-1/PBSF, and its receptor, CXC chemokine receptor 4, as mediators of hematopoiesis. Int J Hematol. 72:408–411. 2000.24). Nakano T, Kodama H, Honjo T. Generation of lymphohematopoietic cells from embryonic stem cells in culture. Science. 265:1098–1101. 1994.
Article25). Nakano T, Kodama H, Honjo T. In vitro development of primitive and definitive erythrocytes from different precursors. Science. 272:722–724. 1996.
Article26). Reubinoff BE, Pera MF, Fong CY, Trounson A, Bongso A. Embryonic stem cell lines from human blastocysts: somatic differentiation in vitro. Nat Biotechnol. 18:399–404. 2000.
Article27). Takahashi T, Yamada K, Tanaka T, Kumano K, Kurokawa M, Hirano N, Honda H, Chiba S, Tsuji K, Yazaki Y, Nakahata T, Hirai H. A potential molecular approach to ex vivo hematopoietic expansion with recombinant epidermal growth factor receptor-expressing adenovirus vector. Blood. 91:4509–4515. 1998.28). Thomson JA, Itskovitz-Eldor J, Shapiro SS, Waknitz MA, Swiergiel JJ, Marshall VS, Jones JM. Embryonic stem cell lines derived from human blastocysts. Science. 282:1145–1147. 1998.
Article29). Trivedi P, Hematti P. Simultaneous generation of CD34(+) primitive hematopoietic cells and CD73(+) mesenchymal stem cells from human embryonic stem cells cocultured with murine OP9 stromal cells. Exp Hematol. 35:146–154. 2007.
Article30). Veiby OP, Jacobsen FW, Cui L, Lyman SD, Jacobsen SE. The flt3 ligand promotes the survival of primitive hemopoietic progenitor cells with myeloid as well as B lymphoid potential. Suppression of apoptosis and counteraction by TNF-alpha and TGF-beta. J Immunol. 157:2953–2960. 1996.31). Vodyanik MA, Bork JA, Thomson JA, Slukvin II. Human embryonic stem cell-derived CD34+ cells: efficient production in the coculture with OP9 stromal cells and analysis of lymphohematopoietic potential. Blood. 105:617–626. 2005.
Article32). Vodyanik MA, Thomson JA, Slukvin II. Leukosialin (CD43) defines hematopoietic progenitors in human embryonic stem cell differentiation cultures. Blood. 108:2095–2105. 2006.
Article33). Zsebo KM, Williams DA, Geissler EN, Broudy VC, Martin FH, Atkins HL, Hsu RY, Birkett NC, Okino KH, Murdock DC. Stem cell factor is encoded at the Sl locus of the mouse and is the ligand for the c-kit tyrosine kinase receptor. Cell. 63:213–224. 1990.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Induction of Dendritic Cell and Cytokine Gene Expression by In situ Delivery of Flt3 Ligand Plasmid
- Effects of Interleukin 4 on the Production of Interleukin 6 in Human Keratinocytes
- Insights into granulosa cell tumors using spontaneous or genetically engineered mouse models
- Generation of an osteoblast-based artificial niche that supports in vitro B lymphopoiesis
- Effects of Thyroid Hormone on Preduction of Interleukin-6 and Interleukin-11 in Human Bone Marrow Stromal Cells