J Korean Endocr Soc.
2006 Oct;21(5):364-369. 10.3803/jkes.2006.21.5.364.
Functional Study of Gene using Inducible Cre System
- Affiliations
-
- 1Department of Molecular Medicine, Kyungpook National University School of Medicine, Korea.
- KMID: 1511984
- DOI: http://doi.org/10.3803/jkes.2006.21.5.364
Abstract
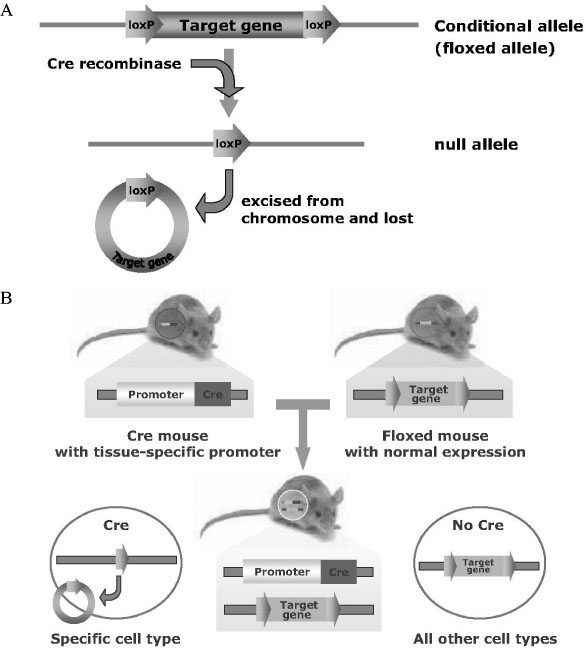
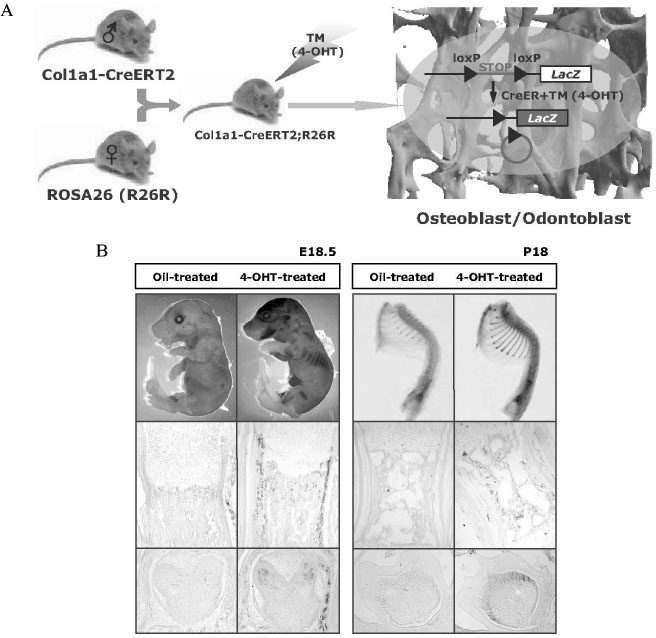
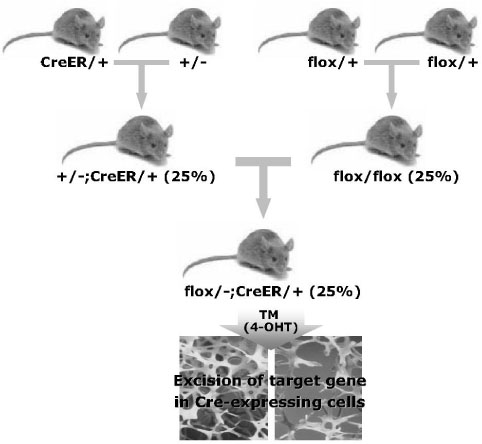
- Gene manipulation by disrupting important genes using homologous recombination in mammals has provided important insights into their function and development with regard to disease. However, many questions related to the genetic pathways that regulate cellular differentiation and function remain to be clarified. In particular, analysis of genetic pathways that control embryonic skeletal development is often hindered by the disruption of critical genes that function in early embryogenesis, thereby leading to embryonic or perinatal death and thus preventing study of the role of these genes in skeletal development and physiology postnatally. To overcome this problem gene-targeting methods, using site- and time-specific recombination based methods with the Cre/loxP system, have been used to delete particular genes in specific tissues and stages during development. Thus, the generation and characterization of transgenic mice expressing Cre recombinase, under the control of a tissue-specific and stage-specific promoter, has become prerequisite for study of the physiology and homeostasis of specific tissues during a specific time frame. In this report, we introduce the principles and methods of site-specific and time-specific recombination using the Cre/loxP and inducible Cre system, and discuss the potential applications for applying this system to the study of the development and physiology of the skeletal system.
MeSH Terms
Figure
Reference
-
1. Rajewsky K, Gu H, Kuhn R, Betz UA, Muller W, Roes J, Schwenk F. Conditional gene targeting. J Clin Invest. 1996. 98:600–603.2. Le Y, Sauer B. Conditional gene knockout using Cre recombinase. Methods Mol Biol. 2000. 136:477–485.3. Sternberg N, Hamilton D. Bacteriophage P1 site-specific recombination. I. Recombination between loxP sites. J Mol Biol. 1981. 150:467–486.4. Indra AK, Warot X, Brocard J, Bornert JM, Xiao JH, Chambon P, Metzger D. Temporally-controlled site-specific mutagenesis in the basal layer of the epidermis: comparison of the recombinase activity of the tamoxifen-inducible Cre-ER(T) and Cre-ER(T2) recombinases. Nucleic Acids Res. 1999. 27:4324–4327.5. Feil R, Wagner J, Metzger D, Chambon P. Regulation of Cre recombinase activity by mutated estrogen receptor ligand-binding domains. Biochem Biophys Res Commun. 1997. 237:752–757.6. Leone DP, Genoud S, Atanasoski S, Grausenburger R, Berger P, Metzger D, Macklin WB, Chambon P, Suter U. Tamoxifen-inducible glia-specific Cre mice for somatic mutagenesis in oligodendrocytes and Schwann cells. Mol Cell Neurosci. 2003. 22:430–440.7. Ducy P, Karsenty G. Two distinct osteoblast-specific cis-acting elements control expression of a mouse osteocalcin gene. Mol Cell Biol. 1995. 15:1858–1869.8. Rossert J, Eberspaecher H, de Crombrugghe B. Separate cis-acting DNA elements of the mouse pro-alpha-1(I) collagen promoter direct expression of reporter genes to different type I collagen-producing cells in transgenic mice. J Cell Biol. 1995. 129:1421–1432.9. Dacquin R, Starbuck M, Schinke T, Karsenty G. Mouse alpha-1(I)-collagen promoter is the best known promoter to drive efficient Cre recombinase expression in osteoblast. Dev Dyn. 2002. 224:245–251.10. Braut A, Kalajzic I, Kalajzic Z, Rowe DW, Kollar EJ, Mina M. Col1a1-GFP transgene expression in developing incisors. Connect Tissue Res. 2002. 43:216–219.11. Kim JE, Kazuhisa N, de Crombrugghe B. Transgenic mice expressing a ligand-inducible cre recombinase in osteoblasts and odontoblasts: a new tool to examine physiology and disease of postnatal bone and tooth. Am J Pathol. 2004. 165:1875–1882.12. Soriano P. Generalized lacZ expression with the ROSA26 Cre reporter strain. Nat Genet. 1999. 21:70–71.13. Nakashima K, Zhou X, Kunkel G, Zhang Z, Deng JM, Behringer RR, de Crombrugghe B. The novel zinc finger-containing transcription factor osterix is required for osteoblast differentiation and bone formation. Cell. 2002. 108:17–29.14. Komori T, Yagi H, Nomura S, Yamaguchi A, Sasaki K, Deguchi K, Shimizu Y, Bronson RT, Go YH, Inada M, Sato M, Okamoto R, Kitamura Y, Yoshiki S, Kishimoto T. Targeted disruption of Cbfa1 results in a complete lack of bone formation owing to maturational arrest of osteoblasts. Cell. 1997. 89:755–764.15. Otto F, Thornell AP, Crompton T, Denzel A, Gilmour KC, Rosewell IR, Stamp GW, Beddington RS, Mundlos S, Olsen BR, Selby PB, Owen MJ. Cbfa1, a candidate gene for cleidocranial dysplasia syndrome, is essential for osteoblast differentiation and bone development. Cell. 1997. 89:765–771.16. Choi JY, Pratap J, Javed A, Zaidi SK, Xing L, Balint E, Dalamangas S, Boyce B, van Wijnen AJ, Lian JB, Stein JL, Jones SN, Stein GS. Subnuclear targeting of Runx/Cbfa/AML factors is essential for tissue-specific differentiation during embryonic development. Proc Natl Acad Sci U S A. 2001. 98:8650–8655.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Tamoxifen-inducible cardiac-specific Cre transgenic mouse using VIPR2 intron
- Establishing a Cre/loxP-based genetic manipulation system for Acanthamoeba: Targeted genome editing and stable reporter expression
- Carbapenem-resistant Enterobacteriaceae in Korea
- Characterization of Cyclic AMP Response Element (CRE) in the Promoter of the Rat Thyrotropin Releasing Hormone (TRH) Gene
- Cell Type-specific Knockout with Gli1-mediated Cre Recombination in the Developing Cerebellum