Ann Clin Microbiol.
2013 Sep;16(3):120-125. 10.5145/ACM.2013.16.3.120.
Loss of blaVIM-2 and blaIMP-1 during the Storage of Gram-Negative Bacilli, Antimicrobial Susceptibility of the Gene-Lost Strain, and Location of the Gene in the Cell
- Affiliations
-
- 1Department of Laboratory Medicine and Research Institute of Bacterial Resistance, Yonsei University College of Medicine, Seoul, Korea. leekcp@yuhs.ac
- 2Department of Laboratory Medicine, National Health Insurance Corporation Ilsan Hospital, Goyang, Korea.
- 3Department of Clinical Laboratory Science, Dong-eui University, Busan, Korea.
- KMID: 1494328
- DOI: http://doi.org/10.5145/ACM.2013.16.3.120
Abstract
- BACKGROUND
Gram-negative bacilli can be stored in cystine tryptic agar (CTA) at room temperature for over 1 year, but we experienced a loss of imipenem resistance among VIM-2-producing isolates. The aims of this study were to determine the frequency of loss of IMP-1 and VIM-2 genes during storage in CTA at room temperature and to document any change in the MIC of antimicrobial agents and the location of the gene.
METHODS
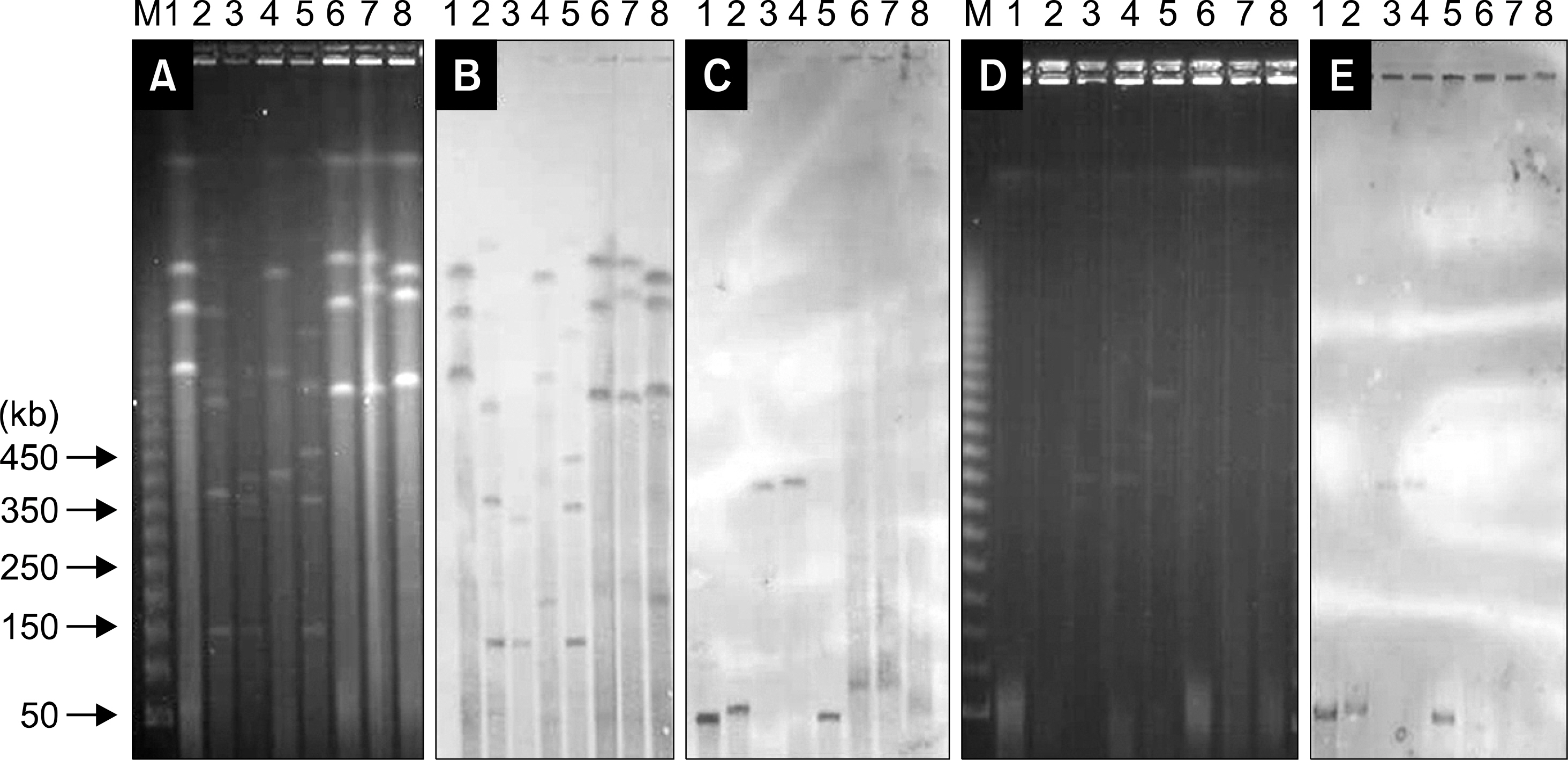
Bacteria were isolated from clinical specimens at Severance Hospital collected from 1995-2000. Modified Hodge and double disk synergy tests were performed for screening of MBL-production isolates, and blaIMP-1 and blaVIM-2 were detected by PCR. Loss of resistance was tested in CTA at room temperature. PFGE and hybridization using a blaVIM-2 probe were carried out to determine the location of the VIM-2 gene.
RESULTS
When VIM-2- and IMP-1-producing strains of eight P. aeruginosa and two Acinetobacter spp. were stored in CTA at room temperature, some isolates lost imipenem resistance after 3 days and 90% lost resistance after 15 weeks. Loss of resistance genes resulted in a decrease of the MIC of imipenem from 32-128 mug/mL to 0.5-8 mug/mL for P. aeruginosa, and from 32 mug/mL to 0.25-4 mug/mL for Acinetobacter spp. Hybridization of I-CeuI and S1-digested and PFGE suggested that VIM-2 genes are located on approximately 50-100 kb or 400 kb plasmids.
CONCLUSION
Isolates may lose resistance genes when stored in CTA at room temperature. Therefore, it is necessary for MBL-production tests including the Modified Hodge test and double disk synergy test and detection of MBL genes.
Keyword
MeSH Terms
Figure
Cited by 1 articles
-
Increasing Carbapenem-Resistant Gram-Negative Bacilli and Decreasing Metallo-β-Lactamase Producers over Eight Years from Korea
Yangsoon Lee, Chang-Ki Kim, Hae-Sun Chung, Dongeun Yong, Seok Hoon Jeong, Kyungwon Lee, Yunsop Chong
Yonsei Med J. 2015;56(2):572-577. doi: 10.3349/ymj.2015.56.2.572.
Reference
-
1.Livingstone D., Gill MJ., Wise R. Mechanisms of resistance to the carbapenems. J Antimicrob Chemother. 1995. 35:1–5.
Article2.Lee K., Lim JB., Yum JH., Yong D., Chong Y., Kim JM, et al. bla VIM-2 cassette-containing novel integrons in metallo-beta-lacta-mase-producing Pseudomonas aeruginosa and Pseudomonas putida isolates disseminated in a Korean hospital. Antimicrob Agents Chemother. 2002. 46:1053–8.3.Lee K., Park AJ., Kim MY., Lee HJ., Cho JH., Kang JO, et al. KONSAR Group. Metallo-beta-lactamase-producing Pseudomonas spp. in Korea: high prevalence of isolates with VIM-2 type and emergence of isolates with IMP-1 type. Yonsei Med J. 2009. 50:335–9.4.Dale J. Extrachromosomal Inheritance. In. Dale JW, editor. Molecular Genetics of Bacteria. 2nd ed. Chichester, England: John Wiley & Sons;1994. p. 133–61.5.Takahashi A., Yomoda S., Kobayashi I., Okubo T., Tsunoda M., Iyobe S. Detection of carbapenemase-producing Acinetobacter baumannii in a hospital. J Clin Microbiol. 2000. 38:526–9.6.Lim Y. Yong D, Yum JH, Lee K, Chong Y. Difficulties in the detection of resistance gene and the determination of imipenem resistance of the metallo-beta-lactamase producing gram-negative bacilli. 42nd Intersci Conf Antimicrob Agents Chemother Abstr. 2002. D-530:139.7.Arakawa Y., Murakami M., Suzuki K., Ito H., Wacharotayankun R., Ohsuka S, et al. A novel integron-like element carrying the metallo- beta-lactamase gene bla IMP. Antimicrob Agents Chemother. 1995. 39:1612–5.8.Poirel L., Naas T., Nicolas D., Collet L., Bellais S., Cavallo JD, et al. Characterization of VIM-2, a carbapenem-hydrolyzing metallo- beta-lactamase and its plasmid- and integron-borne gene from a Pseudomonas aeruginosa clinical isolate in France. Antimicrob Agents Chemother. 2000. 44:891–7.9.Yum JH., Yi K., Lee H., Yong D., Lee K., Kim JM, et al. Molecular characterization of metallo-beta-lactamase-producing Acinetobacter baumannii and Acinetobacter genomospecies 3 from Korea: identification of two new integrons carrying the bla VIM-2 gene cassettes. J Antimicrob Chemother. 2002. 49:837–40.10.Yum JH., Yong D., Lee K., Kim HS., Chong Y. A new integron carrying VIM-2 metallo-beta-lactamase gene cassette in a Serratia marcescens isolate. Diagn Microbiol Infect Dis. 2002. 42:217–9.11.Jeong SH., Lee K., Chong Y., Yum JH., Lee SH., Choi HJ, et al. Characterization of a new integron containing VIM-2, a metallo- beta-lactamase gene cassette, in a clinical isolate of Enterobacter cloacae. J Antimicrob Chemother. 2003. 51:397–400.12.Clinical and Laboratory Standards Institute. Performance Standard for Antimicrobial Susceptibility Testing; Twenty Informational Supplement; Approved Guidelind. Document M100-S14. Wayne, PA; Clinical and Laboratory Standards Institute. 2004.13.Lee K., Lim YS., Yong D., Yum JH., Chong Y. Evaluation of the Hodge test and the imipenem-EDTA double-disk synergy test for differentiating metallo-beta-lactamase-producing isolates of Pseudomonas spp. and Acinetobacter spp. J Clin Microbiol. 2003. 41:4623–9.14.Lee K., Chong Y., Shin HB., Kim YA., Yong D., Yum JH. Modified Hodge and EDTA-disk synergy tests to screen metallo-beta-lacta-mase-producing strains of Pseudomonas and Acinetobacter species. Clin Microbiol Infect. 2001. 7:88–91.15.Riccio ML., Franceschini N., Boschi L., Caravelli B., Cornaglia G., Fontana R, et al. Characterization of the metallo-beta-lactamase determinant of Acinetobacter baumannii AC-54/97 reveals the existence of bla IMP allelic variants carried by gene cassettes of different phylogeny. Antimicrob Agents Chemother. 2000. 44:1229–35.16.Sambrook J., Russell D. Molecular Cloning: A Laboratory Ma-nual. 3rd ed. Cold Spring Harbor (NY); Cold Spring Harbor Laboratory Press. 2001.17.Köhler T., Pechère C. In vitro selection of antibiotic resistance in Pseudomonas aeruginosa. Clin Microbiol Infect. 2001. 7(S):7–10.18.Doménech-Sánchez A., Martínez-Martínez L., Hernández-Allés S., del Carmen Conejo M., Pascual A., Tomás JM, et al. Role of Klebsiella pneumoniae OmpK35 porin in antimicrobial resistance. Antimicrob Agents Chemother. 2003. 47:3332–5.19.Livermore DM., Woodford N. Carbapenemases: a problem in wai-ting? Curr Opin Microbiol. 2000. 3:489–95.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Diversity of Integrons Carrying blaVIM-2 Cassette in Pseudomonas spp. and Acinetobacter spp.
- Antimicrobial Resistance of Clinical Isolates of Acinetobacter spp. Collected from Non-Tertiary Hospitals and Detection of a Metallo-beta-Lactamase-Producing Strain
- Characterization of a New Integron Containing VIM-2 Metallo-beta-Lactamase Gene Cassette in an Isolate of Morganella morganii from a Urine Specimen
- Therapeutic strategy for the management of multidrug-resistant gram-negative bacterial infections
- Prevalence of Metallo-beta-lactamases in Imipenem-non-susceptible Pseudomonas aeruginosa and Acinetobacter baumannii