Korean J Physiol Pharmacol.
2012 Oct;16(5):313-320. 10.4196/kjpp.2012.16.5.313.
The Protective Effect of Eupatilin against Hydrogen Peroxide-Induced Injury Involving 5-Lipoxygenase in Feline Esophageal Epithelial Cells
- Affiliations
-
- 1Department of Pharmacology, College of Pharmacy, Chung-Ang University, Seoul 156-756, Korea. udsohn@cau.ac.kr
- KMID: 1493963
- DOI: http://doi.org/10.4196/kjpp.2012.16.5.313
Abstract
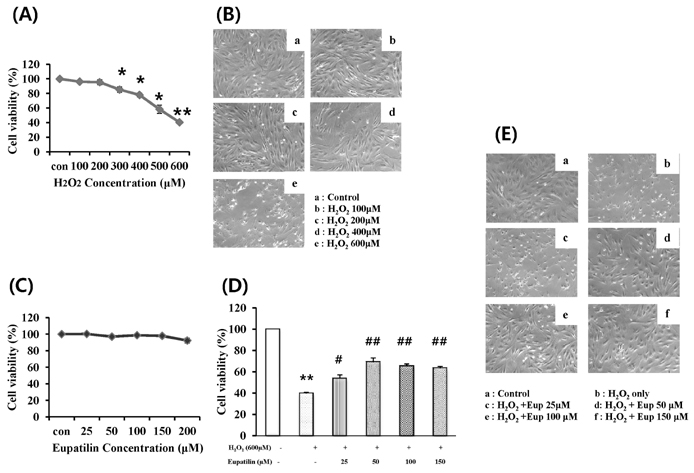
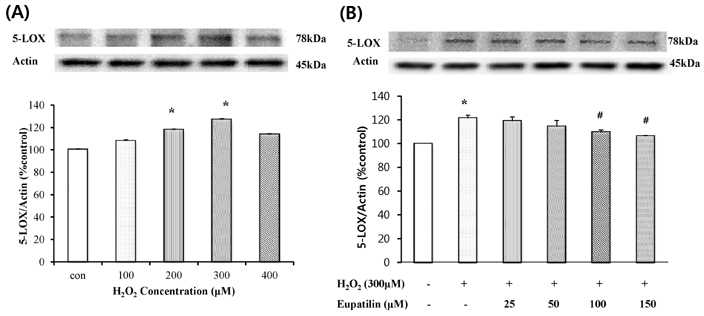
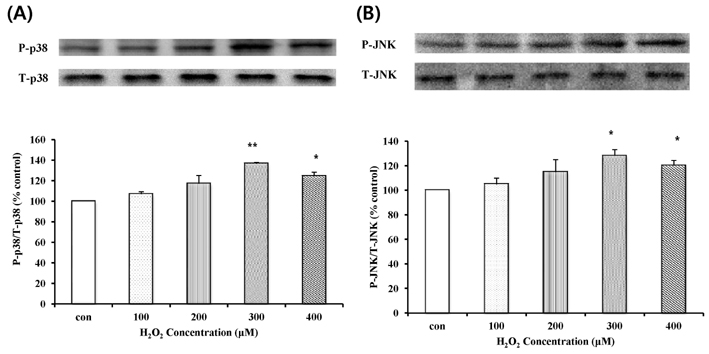
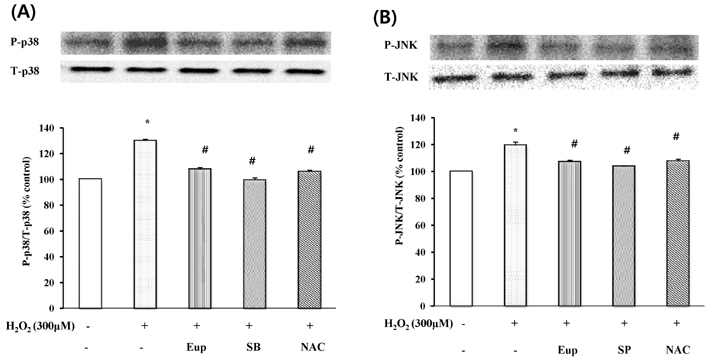
- In this study, we focused to identify whether eupatilin (5,7-dihydroxy-3',4',6-trimethoxyflavone), an extract from Artemisia argyi folium, prevents H2O2-induced injury of cultured feline esophageal epithelial cells. Cell viability was measured by the conventional MTT reduction assay. Western blot analysis was performed to investigate the expression of 5-lipoxygenase by H2O2 treatment in the absence and presence of inhibitors. When cells were exposed to 600 microM H2O2 for 24 hours, cell viability was decreased to 40%. However, when cells were pretreated with 25~150 microM eupatilin for 12 hours, viability was significantly restored in a concentration-dependent manner. H2O2-treated cells were shown to express 5-lipoxygenase, whereas the cells pretreated with eupatilin exhibited reduction in the expression of 5-lipoxygenase. The H2O2-induced increase of 5-lipoxygenase expression was prevented by SB202190, SP600125, or NAC. We further demonstrated that the level of leukotriene B4 (LTB4) was also reduced by eupatilin, SB202190, SP600125, NAC, or nordihydroguaiaretic acid (a lipoxygenase inhibitor) pretreatment. H2O2 induced the activation of p38MAPK and JNK, this activation was inhibited by eupatilin. These results indicate that eupatilin may reduce H2O2-induced cytotoxicity, and 5-lipoxygenase expression and LTB4 production by controlling the p38 MAPK and JNK signaling pathways through antioxidative action in feline esophageal epithelial cells.
MeSH Terms
-
Anthracenes
Arachidonate 5-Lipoxygenase
Artemisia
Blotting, Western
Cell Survival
Epithelial Cells
Flavonoids
Hydrogen
Hydrogen Peroxide
Imidazoles
Leukotriene B4
Lipoxygenase
MAP Kinase Signaling System
Nordihydroguaiaretic Acid
p38 Mitogen-Activated Protein Kinases
Pyridines
Anthracenes
Arachidonate 5-Lipoxygenase
Flavonoids
Hydrogen
Hydrogen Peroxide
Imidazoles
Leukotriene B4
Lipoxygenase
Nordihydroguaiaretic Acid
Pyridines
p38 Mitogen-Activated Protein Kinases
Figure
Cited by 1 articles
-
Cytotoxicity and Structure-activity Relationships of Naphthyridine Derivatives in Human Cervical Cancer, Leukemia, and Prostate Cancer
Yu Jin Hwang, Mi Lyang Chung, Uy Dong Sohn, Chaeuk Im
Korean J Physiol Pharmacol. 2013;17(6):517-523. doi: 10.4196/kjpp.2013.17.6.517.
Reference
-
1. Lee SH, Heo JS, Lee MY, Han HJ. Effect of dihydrotestosterone on hydrogen peroxide-induced apoptosis of mouse embryonic stem cells. J Cell Physiol. 2008. 216:269–275.2. de Magalhães JP, Church GM. Cells discover fire: employing reactive oxygen species in development and consequences for aging. Exp Gerontol. 2006. 41:1–10.3. Menon SG, Goswami PC. A redox cycle within the cell cycle: ring in the old with the new. Oncogene. 2007. 26:1101–1109.4. Lee MN, Lee SH, Lee MY, Kim YH, Park JH, Ryu JM, Yun SP, Lee YJ, Kim MO, Park K, Han HJ. Effect of dihydrotestosterone on mouse embryonic stem cells exposed to H2O2-induced oxidative stress. J Vet Sci. 2008. 9:247–256.5. Lee SH, Na SI, Heo JS, Kim MH, Kim YH, Lee MY, Kim SH, Lee YJ, Han HJ. Arachidonic acid release by H2O2 mediated proliferation of mouse embryonic stem cells: involvement of Ca2+/PKC and MAPKs-induced EGFR transactivation. J Cell Biochem. 2009. 106:787–797.6. Fischer S, Wiesnet M, Renz D, Schaper W. H2O2 induces paracellular permeability of porcine brain-derived microvascular endothelial cells by activation of the p44/42 MAP kinase pathway. Eur J Cell Biol. 2005. 84:687–697.7. Lee WC, Choi CH, Cha SH, Oh HL, Kim YK. Role of ERK in hydrogen peroxide-induced cell death of human glioma cells. Neurochem Res. 2005. 30:263–270.8. Farinati F, Cardin R, Degan P, Rugge M, Mario FD, Bonvicini P, Naccarato R. Oxidative DNA damage accumulation in gastric carcinogenesis. Gut. 1998. 42:351–356.9. Martindale JL, Holbrook NJ. Cellular response to oxidative stress: signaling for suicide and survival. J Cell Physiol. 2002. 192:1–15.10. Kang KA, Lee KH, Zhang R, Piao MJ, Kang MY, Kwak YS, Yoo BS, You HJ, Hyun JW. Protective effects of Castanopsis cuspidate through activation of ERK and NF-kappaB on oxidative cell death induced by hydrogen peroxide. J Toxicol Environ Health A. 2007. 70:1319–1328.11. Olyaee M, Sontag S, Salman W, Schnell T, Mobarhan S, Eiznhamer D, Keshavarzian A. Mucosal reactive oxygen species production in oesophagitis and Barrett's oesophagus. Gut. 1995. 37:168–173.12. Stein HJ, Esplugues J, Whittle BJ, Bauerfeind P, Hinder RA, Blum AL. Direct cytotoxic effect of oxygen radicals on the gastric mucosa. Surgery. 1989. 106:318–323.13. Stein HJ, Hinder RA, Oosthuizen MM. Gastric mucosal injury caused by hemorrhagic shock and reperfusion: protective role of the antioxidant glutathione. Surgery. 1990. 108:467–473.14. Li CT, Zhang WP, Lu YB, Fang SH, Yuan YM, Qi LL, Zhang LH, Huang XJ, Zhang L, Chen Z, Wei EQ. Oxygen-glucose deprivation activates 5-lipoxygenase mediated by oxidative stress through the p38 mitogen-activated protein kinase pathway in PC12 cells. J Neurosci Res. 2009. 87:991–1001.15. Funk CD. Prostaglandins and leukotrienes: advances in eicosanoid biology. Science. 2001. 294:1871–1875.16. Koshihara Y, Neichi T, Murota S, Lao A, Fujimoto Y, Tatsuno T. Selective inhibition of 5-lipoxygenase by natural compounds isolated from Chinese plants, Artemisia rubripes Nakai. FEBS Lett. 1983. 158:41–44.17. Werz O, Steinhilber D. Therapeutic options for 5-lipoxygenase inhibitors. Pharmacol Ther. 2006. 112:701–718.18. Charlier C, Michaux C. Dual inhibition of cyclooxygenase-2 (COX-2) and 5-lipoxygenase (5-LOX) as a new strategy to provide safer non-steroidal anti-inflammatory drugs. Eur J Med Chem. 2003. 38:645–659.19. Brain SD, Williams TJ. Leukotrienes and inflammation. Pharmacol Ther. 1990. 46:57–66.20. Thalhamer T, McGrath MA, Harnett MM. MAPKs and their relevance to arthritis and inflammation. Rheumatology (Oxford). 2008. 47:409–414.21. Tamura S, Hanada M, Ohnishi M, Katsura K, Sasaki M, Kobayashi T. Regulation of stress-activated protein kinase signaling pathways by protein phosphatases. Eur J Biochem. 2002. 269:1060–1066.22. Ichijo H, Nishida E, Irie K, ten Dijke P, Saitoh M, Moriguchi T, Takagi M, Matsumoto K, Miyazono K, Gotoh Y. Induction of apoptosis by ASK1, a mammalian MAPKKK that activates SAPK/JNK and p38 signaling pathways. Science. 1997. 275:90–94.23. Middleton E Jr. Effect of plant flavonoids on immune and inflammatory cell function. Adv Exp Med Biol. 1998. 439:175–182.24. Moreira AJ, Fraga C, Alonso M, Collado PS, Zetller C, Marroni C, Marroni N, González-Gallego J. Quercetin prevents oxidative stress and NF-kappaB activation in gastric mucosa of portal hypertensive rats. Biochem Pharmacol. 2004. 68:1939–1946.25. Middleton E Jr, Kandaswami C, Theoharides TC. The effects of plant flavonoids on mammalian cells: implications for inflammation, heart disease, and cancer. Pharmacol Rev. 2000. 52:673–751.26. Ryu BK, Ahn BO, Oh TY, Kim SH, Kim WB, Lee EB. Studies on protective effect of DA-9601, Artemisia asiatica extract, on acetaminophen- and CCl4-induced liver damage in rats. Arch Pharm Res. 1998. 21:508–513.27. Ahn BO, Ko KH, Oh TY, Cho H, Kim WB, Lee KJ, Cho SW, Hahm KB. Efficacy of use of colonoscopy in dextran sulfate sodium induced ulcerative colitis in rats: the evaluation of the effects of antioxidant by colonoscopy. Int J Colorectal Dis. 2001. 16:174–181.28. Hahm KB, Kim JH, You BM, Kim YS, Cho SW, Yim H, Ahn BO, Kim WB. Induction of apoptosis with an extract of Artemisia asiatica attenuates the severity of cerulein-induced pancreatitis in rats. Pancreas. 1998. 17:153–157.29. Oh TY, Lee JS, Ahn BO, Cho H, Kim WB, Kim YB, Surh YJ, Cho SW, Lee KM, Hahm KB. Oxidative stress is more important than acid in the pathogenesis of reflux oesophagitis in rats. Gut. 2001. 49:364–371.30. Song HJ, Shin CY, Oh TY, Sohn UD. The protective effect of eupatilin on indomethacin-induced cell damage in cultured feline ileal smooth muscle cells: involvement of HO-1 and ERK. J Ethnopharmacol. 2008. 118:94–101.31. Kim do Y, Song HJ, Jeong JH, Suh JS, Sohn UD. Regulation of lysophosphatidic acid-induced COX-2 expression by ERK1/2 activation in cultured feline esophageal epithelial cells. Arch Pharm Res. 2008. 31:1331–1338.32. Park SY, Sohn UD. Inhibitory effect of rosiglitazone on the acid-induced intracellular generation of hydrogen peroxide in cultured feline esophageal epithelial cells. Naunyn Schmiedebergs Arch Pharmacol. 2011. 383:191–201.33. Kim JS, Song HJ, Ko SK, Whang WK, Sohn UD. Quercetin-3-O-beta-d-glucuronopyranoside (QGC)-induced HO-1 expression through ERK and PI3K activation in cultured feline esophageal epithelial cells. Fitoterapia. 2010. 81:85–92.34. Kim N, Cao W, Song IS, Kim CY, Sohn UD, Harnett KM, Biancani P. Leukotriene D4-induced contraction of cat esophageal and lower esophageal sphincter circular smooth muscle. Gastroenterology. 1998. 115:919–928.35. Song HJ, Shin CY, Oh TY, Min YS, Park ES, Sohn UD. Eupatilin with heme oxygenase-1-inducing ability protects cultured feline esophageal epithelial cells from cell damage caused by indomethacin. Biol Pharm Bull. 2009. 32:589–596.36. Amersi F, Buelow R, Kato H, Ke B, Coito AJ, Shen XD, Zhao D, Zaky J, Melinek J, Lassman CR, Kolls JK, Alam J, Ritter T, Volk HD, Farmer DG, Ghobrial RM, Busuttil RW, Kupiec-Weglinski JW. Upregulation of heme oxygenase-1 protects genetically fat Zucker rat livers from ischemia/reperfusion injury. J Clin Invest. 1999. 104:1631–1639.37. Otterbein LE, Kolls JK, Mantell LL, Cook JL, Alam J, Choi AM. Exogenous administration of heme oxygenase-1 by gene transfer provides protection against hyperoxia-induced lung injury. J Clin Invest. 1999. 103:1047–1054.38. Wung BS, Hsu MC, Wu CC, Hsieh CW. Piceatannol upregulates endothelial heme oxygenase-1 expression via novel protein kinase C and tyrosine kinase pathways. Pharmacol Res. 2006. 53:113–122.39. Chen CY, Jang JH, Li MH, Surh YJ. Resveratrol upregulates heme oxygenase-1 expression via activation of NF-E2-related factor 2 in PC12 cells. Biochem Biophys Res Commun. 2005. 331:993–1000.40. Ezekwudo DE, Wang RC, Elegbede JA. Methyl jasmonate induced apoptosis in human prostate carcinoma cells via 5-lipoxygenase dependent pathway. J Exp Ther Oncol. 2007. 6:267–277.41. Li SY, Li Q, Shen JJ, Dong F, Sigmon VK, Liu Y, Ren J. Attenuation of acetaldehyde-induced cell injury by overexpression of aldehyde dehydrogenase-2 (ALDH2) transgene in human cardiac myocytes: role of MAP kinase signaling. J Mol Cell Cardiol. 2006. 40:283–294.42. Lee S, Lee M, Kim SH. Eupatilin inhibits H(2)O(2)-induced apoptotic cell death through inhibition of mitogen-activated protein kinases and nuclear factor-kappaB. Food Chem Toxicol. 2008. 46:2865–2870.43. Nieves D, Moreno JJ. Role of 5-lipoxygenase pathway in the regulation of RAW 264.7 macrophage proliferation. Biochem Pharmacol. 2006. 72:1022–1030.44. Werz O, Bürkert E, Fischer L, Szellas D, Dishart D, Samuelsson B, Rådmark O, Steinhilber D. Extracellular signal-regulated kinases phosphorylate 5-lipoxygenase and stimulate 5-lipoxygenase product formation in leukocytes. FASEB J. 2002. 16:1441–1443.45. Hanaka H, Shimizu T, Izumi T. Stress-induced nuclear export of 5-lipoxygenase. Biochem Biophys Res Commun. 2005. 338:111–116.46. Fiorucci S, Meli R, Bucci M, Cirino G. Dual inhibitors of cyclooxygenase and 5-lipoxygenase. A new avenue in anti-inflammatory therapy? Biochem Pharmacol. 2001. 62:1433–1438.47. Choi EJ, Lee S, Chae JR, Lee HS, Jun CD, Kim SH. Eupatilin inhibits lipopolysaccharide-induced expression of inflammatory mediators in macrophages. Life Sci. 2011. 88:1121–1126.48. Min SW, Kim NJ, Baek NI, Kim DH. Inhibitory effect of eupatilin and jaceosidin isolated from Artemisia princeps on carrageenan-induced inflammation in mice. J Ethnopharmacol. 2009. 125:497–500.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- The Protective Effect of Quercetin-3-O-beta-D-Glucuronopyranoside on Ethanol-induced Damage in Cultured Feline Esophageal Epithelial Cells
- The Effect of Induced Heat Shock Protein 33 in Human Corneal Epithelial Cell
- Effects of Glutathione on Reactive Oxygen Species-Induced Cytotoxicity in Human Retinal Pigment Epithelial Cell Line
- Glutamine Deprivation Causes Hydrogen Peroxide-induced Interleukin-8 Expression via Jak1/Stat3 Activation in Gastric Epithelial AGS Cells
- Effect of Hydrogen Peroxide-induced Oxidative Stress on the Senescence of Trabecular Meshwork Cells