Korean J Physiol Pharmacol.
2008 Apr;12(2):59-64. 10.4196/kjpp.2008.12.2.59.
Relaxant Effect of Spermidine on Acethylcholine and High K+-induced Gastric Contractions of Guinea-Pig
- Affiliations
-
- 1Department of Physiology, Chungbuk National University College of Medicine, Cheongju 361-763, Korea. physiokyc@chungbuk.ac.kr
- 2Department of Physiology and Biophysics, Seoul National University College of Medicine, Seoul 110-799, Korea.
- 3Department of Pharmacology, Chungbuk National University College of Medicine, Cheongju 361-763, Korea.
- 4Department of Physiology, College of Medicine, Shanghai Jiaotong University, 800 Dongchun Rd. Shanghai 200240, P.R. China.
- KMID: 1486144
- DOI: http://doi.org/10.4196/kjpp.2008.12.2.59
Abstract
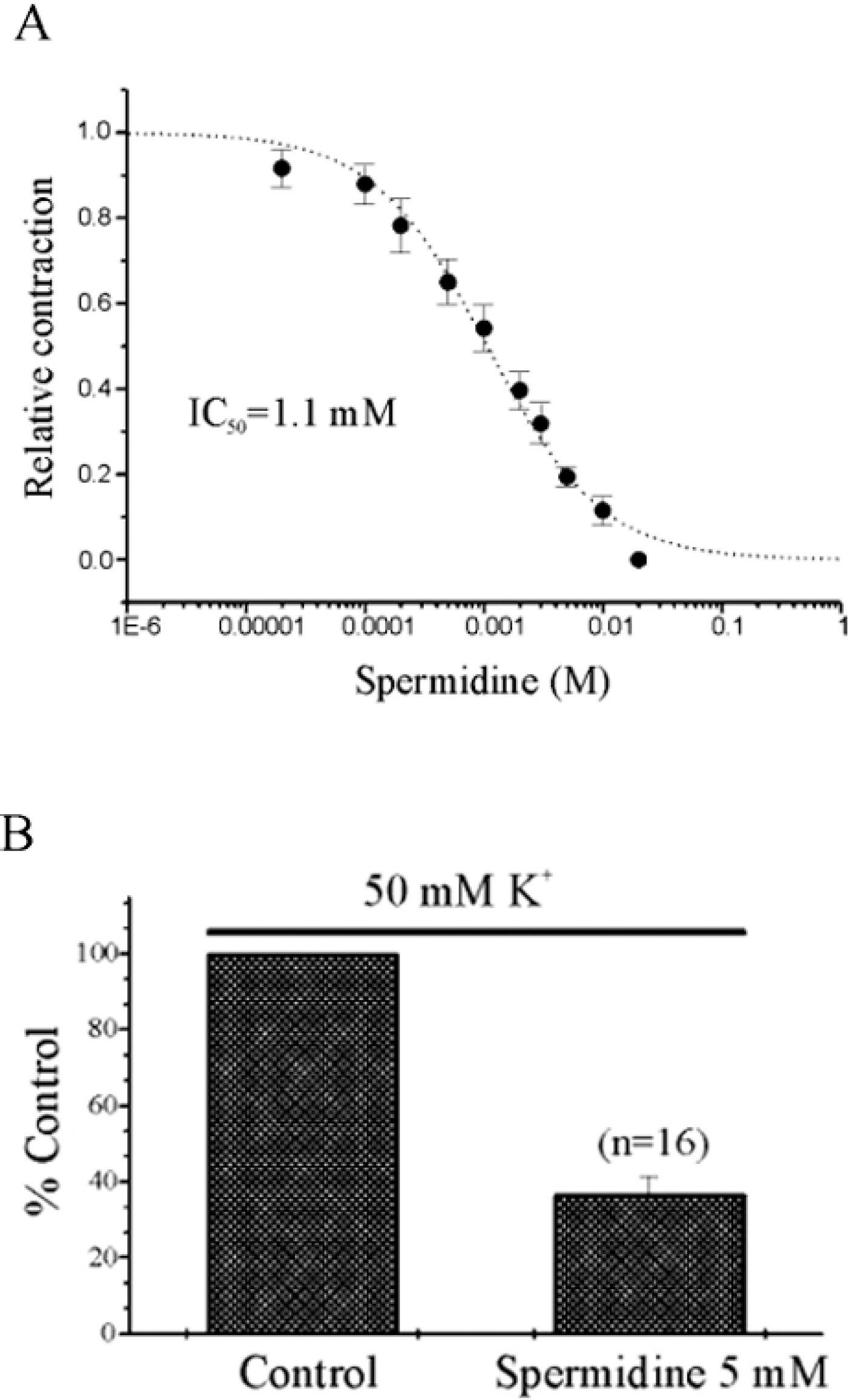
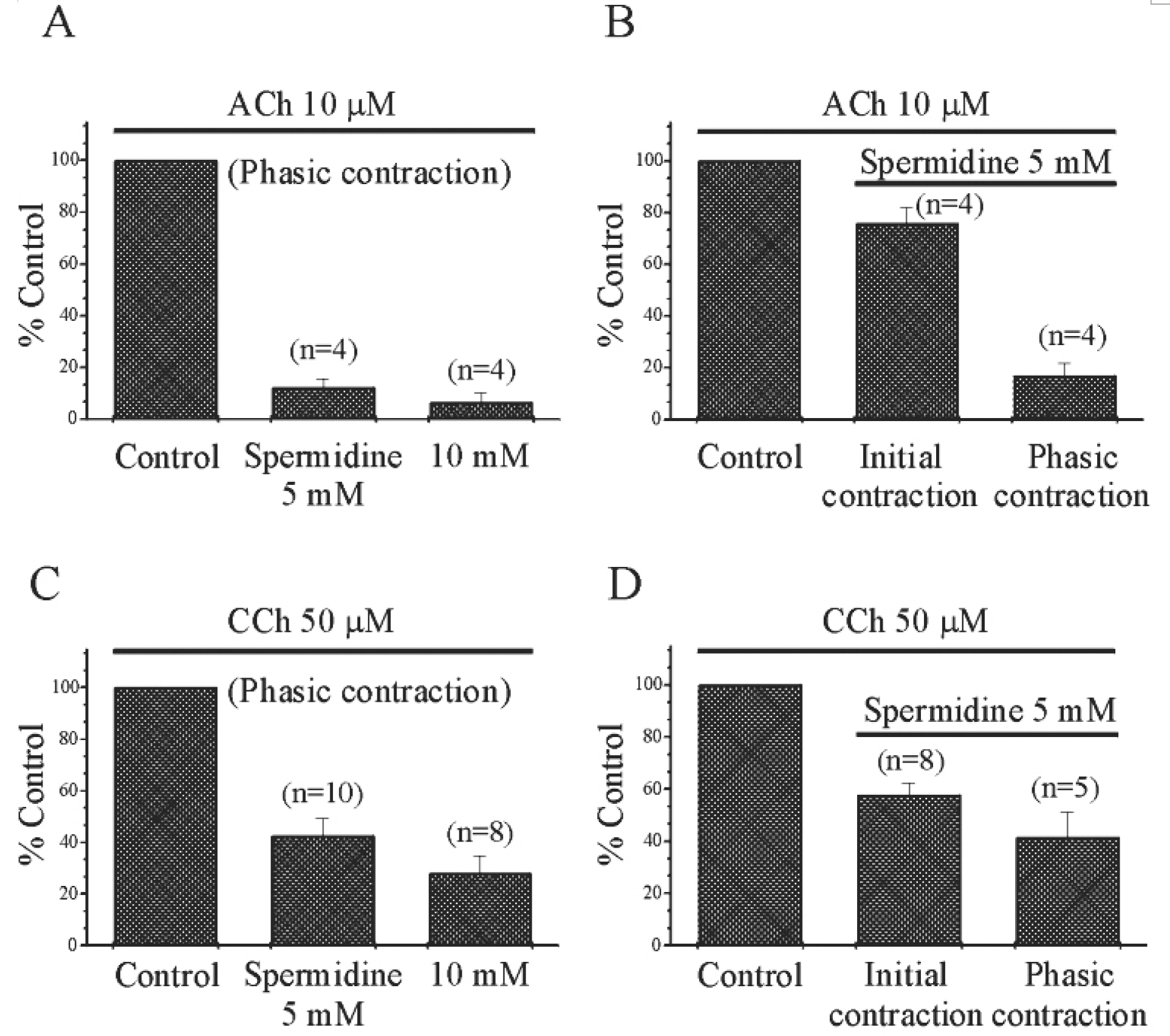
- In our previous study, we found that spermine and putrescine inhibited spontaneous and acetylcholine (ACh)-induced contractions of guinea-pig stomach via inhibition of L-type voltage- dependent calcium current (VDCCL). In this study, we also studied the effect of spermidine on mechanical contractions and calcium channel current (IBa), and then compared its effects to those by spermine and putrescine. Spermidine inhibited spontaneous contraction of the gastric smooth muscle in a concentration-dependent manner (IC50=1.1+/-0.11 mM). Relationship between inhibition of contraction and calcium current by spermidine was studied using 50 mM high K+-induced contraction: Spermidine (5 mM) significantly reduced high K+(50 mM)-induced contraction to 37+/-4.7% of the control (p<0.05), and inhibitory effect of spermidine on IBa was also observed at a wide range of test potential in current/voltage (I/V) relationship. Pre- and post-application of spermidine (5 mM) also significantly inhibited carbachol (CCh) and ACh-induced initial and phasic contractions. Finally, caffeine (10 mM)-induced contraction which is activated by Ca2+-induced Ca2+release (CICR),` was also inhibited by pretreatment of spermidine (5 mM). These findings suggest that spermidine inhibits spontaneous and CCh-induced contraction via inhibition of VDCCL and Ca2+releasing mechanism in guinea-pig stomach.
Keyword
MeSH Terms
Figure
Reference
-
Aihara H., Otomo S., Isobe Y., Ohzeki M., Igarashi K., Hirose S. Polyamine inhibition of gastric ulceration and seretion in rats. Biochem Pharmacol. 32:1733–1736. 1983.Chideckel EW., Fedan JS., Mike P. Polyamines and putrescine relax respiratory tract smooth muscle in the guinea-pig. Eur J Pharmacol. 116:187–190. 1985.De Meis L. Relaxing effect of of spermine and spermidine on intact and glycerol-treated muscle. Am J Physiol. 212:92–96. 1967.Drouin H., Hermann A. Intracellular action of spermine on neuronal Ca2+ and K+ currents. Eur J Neurosci. 6:412–419. 1994.Fernandez AI., Cantabrana B., Sanchez M., Hidalgo A. Extracellular and intracellular effects of polyamines on smooth muscle contractions. Life Sci. 57:855–861. 1995.Gomez M., Hellstrand P. Effects of polyamines on voltage-activated calcium channels in guinea-pig intestinal smooth muscle. Pflügers Arch. 430:501–507. 1995.
ArticleGomez M., Hellstrand P. Endogenous polyamines modulate Ca2+ channel activity in guinea-pig intestinal smooth muscle. Pflügers Arch. 438:445–451. 1999.Hamill OP., Marty A., Neher E., Sakmann B., Sigworth FJ. Improved pathch-clamp techniques for high resolution current recording from cells and cell-free membrane patches. Pflügers Arch. 391:85–100. 1981.Hashimoto H., Unemoto T., Hayashi M. Inhibitory action of spermine on the contractions of rat uterus. Am J Physiol. 225:743–746. 1973.
ArticleHougaard DM., Fujiwara K., Larsson LI. Immunocytochemical localization of polyamines in normal and neoplastic cells. Comparisons to the formaldehyde-fluorescamine and o-phthalal-dehyde methods. Histochem J. 19:643–650. 1987.Igarashi K., Kashiwagi K. Polyamines: mysterious modulators of cellular functions. Biochem Biophys Res Commun. 271:559–564. 2000.
ArticleIino M. Calcium release mechanisms in smooth muscle. Jpn J Pharmacol. 54:345–354. 1990.
ArticleIsenberg G., Klöckner U. Calcium tolerant ventricular myocytes prepared by pre-incubation in a “K-B-medium”. Pflügers Arch. 424:431–438. 1982.Katzka DA., Morad M. Properties of calcium channels in guinea-pig gastric myocytes. J Physiol. 413:175–197. 1989.
ArticleKim SJ., Ahn SC., Kim JK., Kim YC., So I., Kim KW. Changes in intracellular Ca2+ concentration induced by L-type Ca2+ channel current in guinea-pig gastric myocytes. Am J Physiol. 8273:C1947–1956. 1997.Kim SJ., Min KY., Kim YC., Lee SJ., So I., Kim KW. Inhibitory effect of caffeine on carbachol-induced nnonselective cationic current in guinea-pig gastric myocytes. Kor J Physiol Pharmacol. 2:353–359. 1998.Kim YC., Sim JH., Kim YH., Kwon SC., Lee SJ., Kim SR., Kim DW., Park SM., Youn SJ., Lee SJ., Xing de-Gang., Xu WX., Kim KW. Effect of polyamines on contractility of guinea-pig gastric smooth muscle. J Kor Med Sci. 22:48–56. 2007.Lopatin AN., Markhina EN., Nichols CG. Potassium channel block by cytoplasmic polyamines as the mechanism for intrinsic rectification. Nature. 372:366–369. 1994.Martin JS., Tansy MF. Comparison of the inhibitory effects of spermine, papaverine and adrenaline upon isolated segments of the rat small intestine. Clin Exp Pharmacol Physiol 1986. 13:87–90. 1986.
ArticleMaruta K., Mizoguchi Y., Osa T. Effects of polyamines on the mechanical and electrical activities of the isolated circular muscle of rat uterus. Jpn J Physiol. 36:903–915. 1985.
ArticleNagasaki K., Kasai M. Channel selectivity and gating specificity of calcium-induced calcium release channel in isolated sarco-plasmic reticulum. J Biochem. 96:1769–1775. 1984.Nilsson BO., Hellstrand P. Effects of polyamines on intracellular calcium and mechanical activity in smooth muscle of guinea-pig taenia coli. Acta Physiol Scand. 148:37–43. 1993.
ArticleNishimura K., Shiina R., Kashiwagi K., Igarashi K. Decrease in polyamines with aging and their ingestion from food and drink. J Biochem. 139:81–90. 2006.
ArticlePalade P. Drug induced calcium release from isolated sarcoplasmic reticulum. III. Block of calcium-induced calcium release by organic polyamines. J Biol Chem. 262:6149–6154. 1987.Peulen O., Deloyer P., Dandrifosse G. Short-term effects of spermine ingestion on the small intestine: a comparison of suckling and weaned rats. Reprod Nutr Dev. 44:353–364. 2004.
ArticleSato K., Sanders KM., Gerthoffer WT., Publicover NG. Sources of calcium utilized in cholinergic responses in canine colonic smooth muscle. Am J Physiol. 267:C1666–C1673. 1994.
ArticleSchoemaker H. Polyamines allosterically modulate [3H] nitrendipine binding to the voltage-sensitive calcium in rat brain. Eur J Pharmacol. 225:167–169. 1992.Swärd K., Nillsson B-O., Hellstrand P. Polyamines increase Ca2+ sensitivity in permeabilized smooth muscle of guinea-pig ileum. Am J Physiol. 266:C1754–C1763. 1994.Tabor CW., Tabor H. Polyamines. Annu Rev Biochem. 53:749–790. 1984.
ArticleTansy MF., Martin JS., Landin WF., Kendall FM., Melamed S. Spermine and spermidine as inhibitors of gastrointestinal motor activity. Surg Gynecol Obstet. 154:74–80. 1982.Tsvilovskyy W., Zholos AV., Bolton TB. Effects of polyamines on the muscarinic receptor-operated cation current in guinea-pig ileal smooth muscle myocytes. Br J Pharmacol. 143:968–975. 2004.
ArticleWery I., Kaouass M., Deloyer O., Buts J-P., Barbason H., Dandrifosse G. Exogenous spermine induces maturation of the liver in suckling rats. Hepatology. 24:1206–1210. 1996.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Forskolin Changes the Relationship between Cytosolic Ca2+ and Contraction in Guinea Pig Ileum
- Effects of Polyamines on Contractility of Guinea-Pig Gastric Smooth Muscle
- Exophthalmos Inhibitory Effect of Anti-EPS Serum
- Neurotensin enhances gastric motility in antral circular muscle strip of guinea-pig.
- The Effect of Enflurane on the Tracheal Smooth Muscle Contracted with Electrical Field Stimulation in the Guinea Pig