Infect Chemother.
2009 Oct;41(5):272-278. 10.3947/ic.2009.41.5.272.
Seroepidemiology of Hepatitis A among Healthcare Workers and Their Response to Vaccination Recommendation at a Korean Hospital
- Affiliations
-
- 1Department of Internal Medicine, Dongguk University College of Medicine, Goyang, Korea. yonathan@hanafos.com
- 2Department of Laboratory Medicine, Dongguk University College of Medicine, Goyang, Korea.
- 3Infection Control Office, Dongguk University Ilsan Hospital, Goyang, Korea.
- KMID: 1473652
- DOI: http://doi.org/10.3947/ic.2009.41.5.272
Abstract
- BACKGROUND
Recently, the incidence of hepatitis A has increased in Korea and an outbreak among healthcare workers (HCWs) has also been reported. This study was performed to evaluate the seroepidemiology of hepatitis A among HCWs and their response to vaccination recommendation at a Korean hospital.
MATERIALS AND METHODS
HCWs aged 20-39 years were tested for IgG antibodies against hepatitis A virus (HAV) using ARCHITECT HAVAb-IgG (Abbott Diagnostics Division, Wiesbaden, Germany) during July, 2008. Vaccination was recommended for the seronegative HCWs. Data on age, sex, place of birth, number of siblings, number of children, travel history to endemic areas, occupations, and vaccination history were collected. Statistical analyses were conducted to identify variables related to HAV seropositivity.
RESULTS
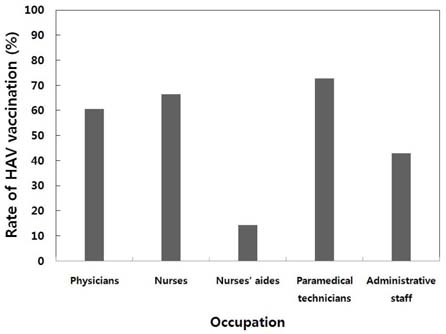
Among a total of 391 HCWs enrolled, 75 (19%) were males and 316 (81%) were females. The percentages of HCWs aged 20 to 24 years, 25 to 29 years, 30 to 34 years, and 35 to 39 years were 23%, 48%, 17%, and 12%, respectively. The study population comprised of physicians (11%), nurses (62%), nurse aides (5%), paramedical technicians (13%), and administrative staff (9%). Seropositivity for HAV significantly increased with age (P<0.05):1.1% for 20-24, 8.6% for 25-29, 35.8% for 30-34, and 60.9% for 35-39 years-of-age. Multivariate analysis revealed that HAV seroprevalence correlated significantly with age, HCWs born in the non-metropolitan areas, and having > or = 3 children (P<0.05). The types of work the HCWs do at the hospital were not significantly associated with HAV seropositivity in multivariate analyses. Of a total of 322 seronegative HCWs, 121 (38%) were not vaccinated in spite of recommendation. The reasons for non-compliance were lack of understanding on the necessity of vaccination (39%), lack of time (26%), expensive costs (16%), fear of injection (15%), and some other reasons including pregnancy (4%).
CONCLUSIONS
Since the seropositivity for HAV is low, vaccination against Hepatitis A should be considered for all HCWs aged 20-39 years in Korea. Education on the necessity of vaccination is warranted to increase compliance.
Keyword
MeSH Terms
-
Aged
Antibodies
Child
Compliance
Delivery of Health Care
Female
Hepatitis
Hepatitis A
Hepatitis A virus
Humans
Hypogonadism
Immunoglobulin G
Incidence
Korea
Male
Mitochondrial Diseases
Multivariate Analysis
Nurses' Aides
Occupations
Ophthalmoplegia
Pregnancy
Residence Characteristics
Seroepidemiologic Studies
Siblings
Vaccination
Antibodies
Hypogonadism
Immunoglobulin G
Mitochondrial Diseases
Ophthalmoplegia
Figure
Cited by 1 articles
-
Seroprevalence of Anti-hepatitis B Virus, Anti-hepatitis A Virus, and Anti-varicella Zoster Virus Antibodies in Nursing Students from 2009 to 2013
Park Jin-Hee, Shon Joung-A
Korean J Nosocomial Infect Control. 2013;21(1):31-36. doi: 10.14192/kjnlc.2016.21.1.31.
Reference
-
1. Cuthbert JA. Hepatitis A: old and new. Clin Microbiol Rev. 2001. 14:38–58.
Article2. Brundage SC, Fitzpatrick AN. Hepatitis A. Am Fam Physician. 2006. 73:2162–2168.3. Headler SC, Webster HM, Erben JJ, Swanson JE, Maynard JE. Hepatitis A in day-care centers. A community-wide assessment. N Eng J Med. 1980. 302:1222–1227.4. Lednar WM, Lemon SM, Kirkpatrick JW, Redfield RR, Fields ML, Kelley PW. Frequency of illness associated with epidemic hepatitis A virus infections in adults. Am J Epidemiol. 1985. 122:226–233.
Article5. Jeong SH, Hwang SG, Park SJ, Kang SK, Jee Y. Current status of acute viral hepatitis in Korea; epidemiology, clinical, virological characteristics, and effects of antiviral treatment in severe acute hepatitis B and C-intrim report. Korean J Hepatol. 2007. 13:Suppl 4. 101–107.6. Van Damme P, Thoelen S, Van der Auwera JC, Bare R, Meheus A. Hagberg M, Hofmann F, StoBel U, Westlander G, editors. Viral hepatitis among health care workers - epidemiology and prevention. Occupational health for health care workers. 1993. Landsberg/Lech: Ecomed;133–137.7. Hofmann F, Wehrle G, Berthold H, Köster D. Hepatitis A as an occupational hazard. Vaccine. 1992. 10:Suppl 1. S82–S84.
Article8. Chodick G, Ashkenazi S, Lerman Y. The risk of hepatitis A infection among health care workers: a review of reported outbreaks and sero-epidemiologic studies. J Hosp Infect. 2006. 62:414–420.
Article9. Lerman Y, Chodik G, Aloni H, Ribak J, Ashkenazi S. Occupations at increased risk of hepatitis A: a 2-year nationwide historical prospective study. Am J Epidemiol. 1999. 150:312–320.
Article10. Park JY, Lee JB, Jeong SY, LEE SH, Lee MA, Choi HJ. Molecular characterization of an acute hepatitis A outbreak among healthcare workers at Korean hospital. J Hosp Infect. 2007. 67:175–181.
Article11. Song YB, Lee JH, Choi MS, Koh KC, Paik SW, Yoo BC, Choi YH, Sohn HJ, Lee KH, Rhee JC. The age-specific seroprevalence of hepatitis A virus antibody in Korea. Korean J Hepatol. 2007. 13:27–33.12. Feinstone SM. Hepatitis A: epidemiology and prevention. Eur J Gastroenterol Hepatol. 1996. 8:300–305.13. Shapiro CN, Margolis HS. Worldwide epidemiology of hepatitis A virus infection. J Hepatol. 1993. 18:Suppl 2. S11–S14.
Article14. Steffen R. Risk of hepatitis A in travellers. Vaccine. 1992. 10:Suppl 1. S69–S72.
Article15. Corey L, Holmes KK. Sexual transmission of hepatitis A in homosexual men: incidence and mechanism. N Engl J Med. 1980. 302:435–438.
Article16. Holter E, Siebke JC. Hepatitis A in young Norwegian drug addicts and prison inmates. Infection. 1988. 16:91–94.
Article17. Bell BP, Shapiro CN, Alter MJ, Moyer LA, Judson FN, Mottram K, Fleenor M, Ryder PL, Margolis HS. The diverse patterns of hepatitis A epidemiology in the United States-implications for vaccination strategies. J Infect Dis. 1998. 178:1579–1584.
Article18. Kim CY, Hong WS. Seroepidemiology of type A and type B hepatitis in Seoul area. Korean J Intern Med. 1982. 25:19–27.19. Lee JI, Kim JY, Kim ST, Yoo SY, Chung SM, Kim YK, Lee BH. Epidemiologic study of antibody to hepatitis A antigen in Choong Chung area. Korean J Gastroenterol. 1982. 14:87–91.20. Kim TW, Lee KJ. Antibody of hepatitis A antigen in children and adolescents in Korea. J Korean Pediatr Soc. 1982. 25:36–40.21. Lee KY, Song KH, Kang JH. Seroepidemiology of hepatitis A in Taejon, Korea 1996. J Korean Pediatr Soc. 1998. 41:53–61.22. Gelber SE, Ratner AJ. Hospital-acquired viral pathogens in the neonatal intensive care unit. Semin Perinatol. 2002. 26:346–356.
Article23. Doebbeling BN, Li N, Wenzel RP. An outbreak of hepatitis A among health care workers: risk factors for transmission. Am J Public Health. 1993. 83:1679–1684.
Article24. Gastmeier P, Stamm-Balderjahn S, Hansen S, Nitzschke-Tiemann F, Zuschneid I, Groneberg K, Rüden H. How outbreaks can contribute to prevention of nosocomial infection: analysis of 1,022 outbreaks. Infect Control Hosp Epidemiol. 2005. 26:357–361.
Article25. Skidmore SJ, Gully PR, Middleton JD, Hassam ZA, Singal GM. An outbreak of hepatitis A on a hospital ward. J Med Virol. 1985. 17:175–177.
Article26. Chodick G, Ashkenazi S, Aloni H, Peled T, Lerman Y. Hepatitis A virus seropositivity among hospital and community healthcare workers in Israel-the role of occupation, demography and socioeconomic background. J Hosp Infect. 2003. 54:135–140.
Article27. Clemens R, Safary A, Hepburn A, Roche C, Stanbury WJ, André FE. Clinical experience with an inactivated hepatitis A vaccine. J Infect Dis. 1995. 171:Suppl 1. S44–S49.
Article28. Ashur Y, Adler R, Rowe M, Shouval D. Comparison of immunogenicity of two hepatitis A vaccines--VAQTA and HAVRIX--in young adults. Vaccine. 1999. 17:2290–2296.
Article29. McMahon BJ, Williams J, Bulkow L, Snowball M, Wainwright R, Kennedy M, Krause D. Immunogenicity of an inactivated hepatitis A vaccine in Alaska Native children and Native and non-Native adults. J Infect Dis. 1995. 171:676–679.
Article30. Van Damme P, Banatvala J, Fay O, Iwarson S, McMahon B, Van Herck K, Shouval D, Bonanni P, Connor B, Cooksley G, Leroux-Roels G, Von Sonnenburg F. International Consensus Group on Hepatitis A Virus Immunity. Hepatitis A booster vaccination: is there a need? Lancet. 2003. 362:1065–1071.
Article31. Van Herck K, Van Damme P. Inactivated hepatitis A vaccine-induced antibodies: follow-up and estimates of long-term persistence. J Med Virol. 2001. 63:1–7.
Article32. Korean Society of Pediatrics. Hepatitis A, Guidelines for vaccination. 2002. 5th ed. Seoul: Kwangmoon Co;223.33. The Korean Society of Infectious Diseases. Vaccination for adult. 2007. 1st ed. Seoul: Koonja Publishing Co.;46.34. Kim JS, Baek YS, Chung MH, Lee JS, Oh KS. The pattern of vaccine administration accessed by vaccine consumption in a university hospital. Infect Chemother. 2008. 40:154–161.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Seroprevalence of Hepatitis A Virus Antibody and Vaccination among Healthcare Workers in a Teaching Hospital
- Hepatitis A Vaccine
- Hepatitis B vaccination coverage and the determinants of vaccination among health care workers in selected health facilities in Lusaka district, Zambia: an exploratory study
- Seroepidemiology of Hepatitis A and Hepatitis B in Korean Children
- Prevention of Viral Hepatitis and Vaccination