Immune Netw.
2011 Aug;11(4):210-215. 10.4110/in.2011.11.4.210.
The Anti-tumor Activity of Vitamin C via the Increase of Fas (CD95) and MHC I Expression on Human Stomach Cancer Cell Line, SNU1
- Affiliations
-
- 1Department of Anatomy and Tumor Immunity Medical Research Center, Seoul National University College of Medicine, Seoul 110-799, Korea. genius29@snu.ac.kr, kinglee@snu.ac.kr
- 2University of Illinois at Urbana Champaign, USA.
- 3Undergraduate School, University of Wisconsin-Madison, USA.
- 4Institute of Complementary and Integrative Medicine, Medical Research Center, Seoul National University, Seoul 110-799, Korea.
- KMID: 1449812
- DOI: http://doi.org/10.4110/in.2011.11.4.210
Abstract
- BACKGROUND
It is already known that high concentration of vitamin C induces apoptosis on tumor cells. However, there is no report regarding the function of vitamin C on the modulation of immune susceptibility of cancer. Therefore, we investigated whether vitamin C can modulate immune susceptibility of tumor cells, especially on the induction of Fas-mediated apoptosis.
METHODS
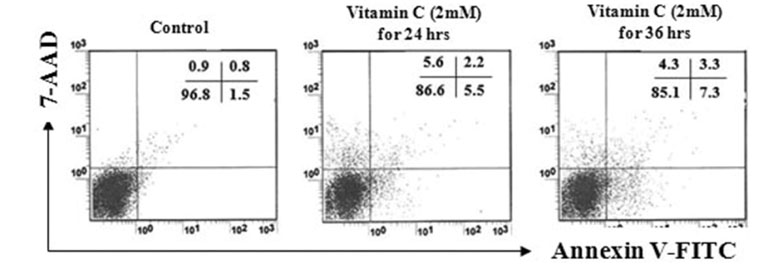
First, the optimal concentration of vitamin C, which cannot induce damages on tumor cells for 36 hrs. We found that 2 mM of vitamin C did not show harmful effect. In addition, the optimal concentration of agonistic anti-Fas Abs for 18 hrs was examined.
RESULTS
As a result, 400 ng/ml of agonistic anti-Fas Abs did not induce apoptosis on tumor cells. Next, we tried to find the effect of 2 mM of vitamin C on the modulation of the susceptibility to agonistic anti-Fas Abs. When tumor cells were cultured with 400 ng/ml of agonistic anti-Fas Abs for 18 hrs, after pre-treatment with 2 mM of vitamin C for 24 hrs, viability of cells was decreased. Interestingly, we found that the expression of Fas (CD95) and MHC class I was increased by the treatment of vitamin C.
CONCLUSION
Taken together, vitamin C increases the susceptibility of tumor cells to anti-Fas Abs and the expression of Fas (CD95) and MHC class I on tumor cells.
Keyword
MeSH Terms
Figure
Cited by 1 articles
-
Vitamin C Up-regulates Expression of CD80, CD86 and MHC Class II on Dendritic Cell Line, DC-1 Via the Activation of p38 MAPK
Hyung Woo Kim, Su In Cho, Seyeon Bae, Hyemin Kim, Yejin Kim, Young-il Hwang, Jae Seung Kang, Wang Jae Lee
Immune Netw. 2012;12(6):277-283. doi: 10.4110/in.2012.12.6.277.
Reference
-
1. Kang JS, Cho D, Kim YI, Hahm E, Yang Y, Kim D, Hur D, Park H, Bang S, Hwang YI, Lee WJ. L-ascorbic acid (vitamin C) induces the apoptosis of B16 murine melanoma cells via a caspase-8-independent pathway. Cancer Immunol Immunother. 2003. 52:693–698.
Article2. Kang JS, Cho D, Kim YI, Hahm E, Kim YS, Jin SN, Kim HN, Kim D, Hur D, Park H, Hwang YI, Lee WJ. Sodium ascorbate (vitamin C) induces apoptosis in melanoma cells via the down-regulation of transferrin receptor dependent iron uptake. J Cell Physiol. 2005. 204:192–197.
Article3. Kim JE, Jin DH, Lee SD, Hong SW, Shin JS, Lee SK, Jung DJ, Kang JS, Lee WJ. Vitamin C inhibits p53-induced replicative senescence through suppression of ROS production and p38 MAPK activity. Int J Mol Med. 2008. 22:651–655.
Article4. Hahm E, Jin DH, Kang JS, Kim YI, Hong SW, Lee SK, Kim HN, Jung da J, Kim JE, Shin DH, Hwang YI, Kim YS, Hur DY, Yang Y, Cho D, Lee MS, Lee WJ. The molecular mechanisms of vitamin C on cell cycle regulation in B16F10 murine melanoma. J Cell Biochem. 2007. 102:1002–1010.
Article5. Lee SK, Kang JS, Jung da J, Hur DY, Kim JE, Hahm E, Bae S, Kim HW, Kim D, Cho BJ, Cho D, Shin DH, Hwang YI, Lee WJ. Vitamin C suppresses proliferation of the human melanoma cell SK-MEL-2 through the inhibition of cyclooxygenase-2 (COX-2) expression and the modulation of insulin-like growth factor II (IGF-II) production. J Cell Physiol. 2008. 216:180–188.
Article6. Kim HN, Kim H, Kong JM, Bae S, Kim YS, Lee N, Cho BJ, Lee SK, Kim HR, Hwang YI, Kang JS, Lee WJ. Vitamin C down-regulates VEGF production in B16F10 murine melanoma cells via the suppression of p42/44 MAPK activation. J Cell Biochem. 2011. 112:894–901.
Article7. Cho D, Hahm E, Kang JS, Kim YI, Yang Y, Park JH, Kim D, Kim S, Kim YS, Hur D, Park H, Pang S, Hwang YI, Lee WJ. Vitamin C downregulates interleukin-18 production by increasing reactive oxygen intermediate and mitogen-activated protein kinase signalling in B16F10 murine melanoma cells. Melanoma Res. 2003. 13:549–554.
Article8. Hong SW, Jin DH, Hahm ES, Yim SH, Lim JS, Kim KI, Yang Y, Lee SS, Kang JS, Lee WJ, Lee WK, Lee MS. Ascorbate (vitamin C) induces cell death through the apoptosis-inducing factor in human breast cancer cells. Oncol Rep. 2007. 18:811–815.
Article9. Ferrone S, Marincola FM. Loss of HLA class I antigens by melanoma cells: molecular mechanisms, functional significance and clinical relevance. Immunol Today. 1995. 16:487–494.
Article10. Hahne M, Rimoldi D, Schröter M, Romero P, Schreier M, French LE, Schneider P, Bornand T, Fontana A, Lienard D, Cerottini J, Tschopp J. Melanoma cell expression of Fas (Apo-1/CD95) ligand: implications for tumor immune escape. Science. 1996. 274:1363–1366.
Article11. Krammer PH. CD95's deadly mission in the immune system. Nature. 2000. 407:789–795.
Article12. Kroemer G, Zamzami N, Susin SA. Mitochondrial control of apoptosis. Immunol Today. 1997. 18:44–51.
Article13. Rosenberg SA, Lotze MT, Muul LM, Leitman S, Chang AE, Ettinghausen SE, Matory YL, Skibber JM, Shiloni E, Vetto JT, Seipp CA, Simpson C, Reichert CM. Observations on the systemic administration of autologous lymphokine-activated killer cells and recombinant interleukin-2 to patients with metastatic cancer. N Engl J Med. 1985. 313:1485–1492.
Article14. West WH, Tauer KW, Yannelli JR, Marshall GD, Orr DW, Thurman GB, Oldham RK. Constant-infusion recombinant interleukin-2 in adoptive immunotherapy of advanced cancer. N Engl J Med. 1987. 316:898–905.
Article15. Thurner B, Haendle I, Röder C, Dieckmann D, Keikavoussi P, Jonuleit H, Bender A, Maczek C, Schreiner D, von den Driesch P, Bröcker EB, Steinman RM, Enk A, Kämpgen E, Schuler G. Vaccination with mage-3A1 peptide-pulsed mature, monocyte-derived dendritic cells expands specific cytotoxic T cells and induces regression of some metastases in advanced stage IV melanoma. J Exp Med. 1999. 190:1669–1678.
Article16. Nestle FO, Alijagic S, Gilliet M, Sun Y, Grabbe S, Dummer R, Burg G, Schadendorf D. Vaccination of melanoma patients with peptide- or tumor lysate-pulsed dendritic cells. Nat Med. 1998. 4:328–332.
Article17. Siegel BV, Morton JI. Vitamin C and immunity: natural killer (NK) cell factor. Int J Vitam Nutr Res. 1983. 53:179–183.18. Jeong YJ, Hong SW, Kim JH, Jin DH, Kang JS, Lee WJ, Hwang YI. Vitamin C-treated murine bone marrow-derived dendritic cells preferentially drive naïve T cells into Th1 cells by increased IL-12 secretions. Cell Immunol. 2011. 266:192–199.
Article19. Levine M, Conry-Cantilena C, Wang Y, Welch RW, Washko PW, Dhariwal KR, Park JB, Lazarev A, Graumlich JF, King J, Cantilena LR. Vitamin C pharmacokinetics in healthy volunteers: evidence for a recommended dietary allowance. Proc Natl Acad Sci U S A. 1996. 93:3704–3709.
Article20. Levine M, Wang Y, Padayatty SJ, Morrow J. A new recommended dietary allowance of vitamin C for healthy young women. Proc Natl Acad Sci U S A. 2001. 98:9842–9846.
Article21. Padayatty SJ, Sun H, Wang Y, Riordan HD, Hewitt SM, Katz A, Wesley RA, Levine M. Vitamin C pharmacokinetics: implications for oral and intravenous use. Ann Intern Med. 2004. 140:533–537.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- The effect of cyclophosphamide on Fas-mediated apoptosis
- Changes of phospholipase D activity in TNF-alpha and anti-Fas/Apo1 monoclonal antibody induced apoptosis in HL-60 and A20 cells
- Sensitivity of CD95-induced apoptosis in different proliferative status of human retinal pigment epithelial cells
- Effect of Interferon-r on Fas-mediated Apoptosis of Colon Cancer Cell Line HT-29
- Expression of dendritic cell markers on cultured neutrophils and its modulation by anti-apoptotic and pro-apoptotic compounds