Anat Cell Biol.
2011 Dec;44(4):274-283. 10.5115/acb.2011.44.4.274.
Morphological evidences in circumvallate papilla and von Ebners' gland development in mice
- Affiliations
-
- 1School of Life Science and Biotechnology, Institute for Hard Tissue and Bio-tooth Regeneration, School of Dentistry, Kyungpook National University, Daegu, Korea.
- 2Department of Biochemistry, Institute for Hard Tissue and Bio-tooth Regeneration, School of Dentistry, Kyungpook National University, Daegu, Korea. jykim91@knu.ac.kr
- 3Department of Oral and Maxillofacial Radiology, Institute for Hard Tissue and Bio-tooth Regeneration, School of Dentistry, Kyungpook National University, Daegu, Korea.
- 4Department of Anatomy and Neuroscience, Institute for Hard Tissue and Bio-tooth Regeneration, School of Dentistry, Kyungpook National University, Daegu, Korea.
- 5Dental Education Development Center, Tokyo Dental College, Chiba, Japan.
- KMID: 1447441
- DOI: http://doi.org/10.5115/acb.2011.44.4.274
Abstract
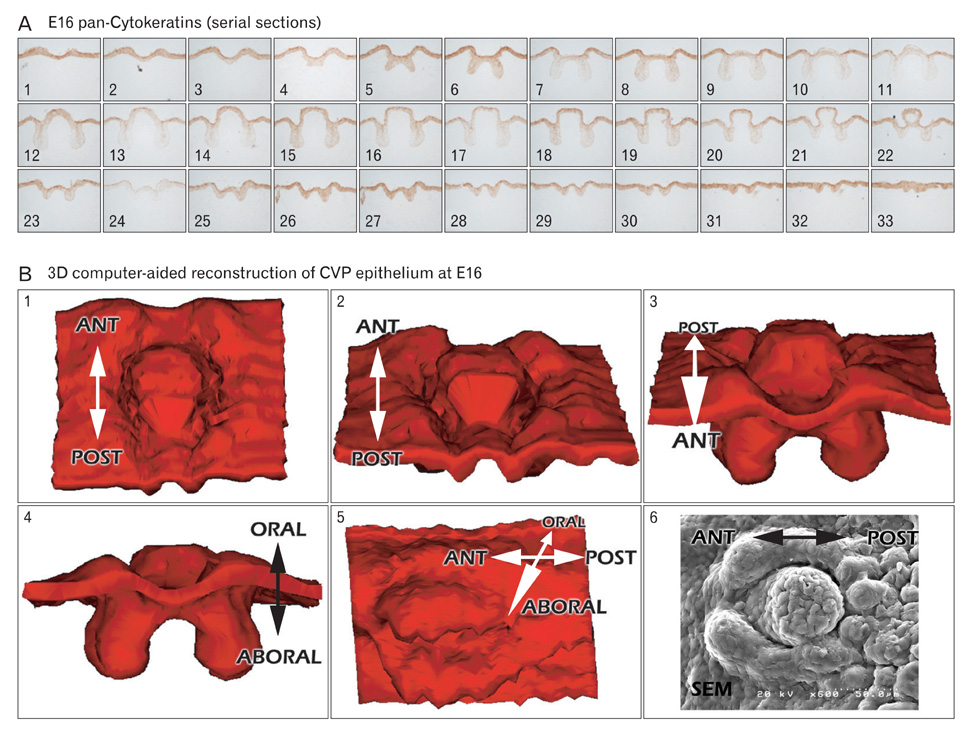
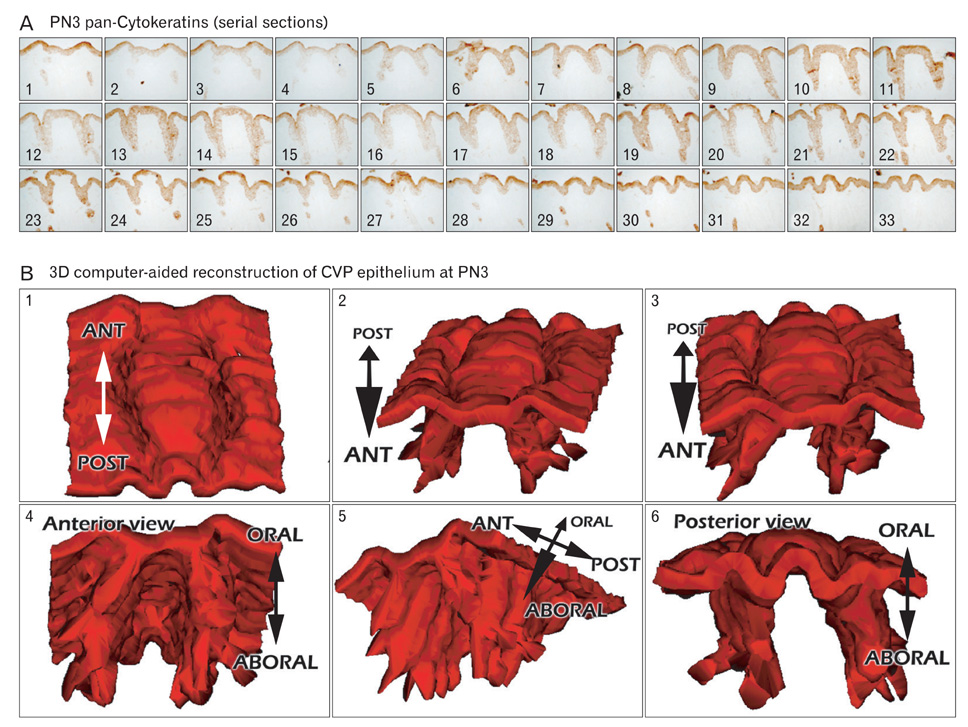
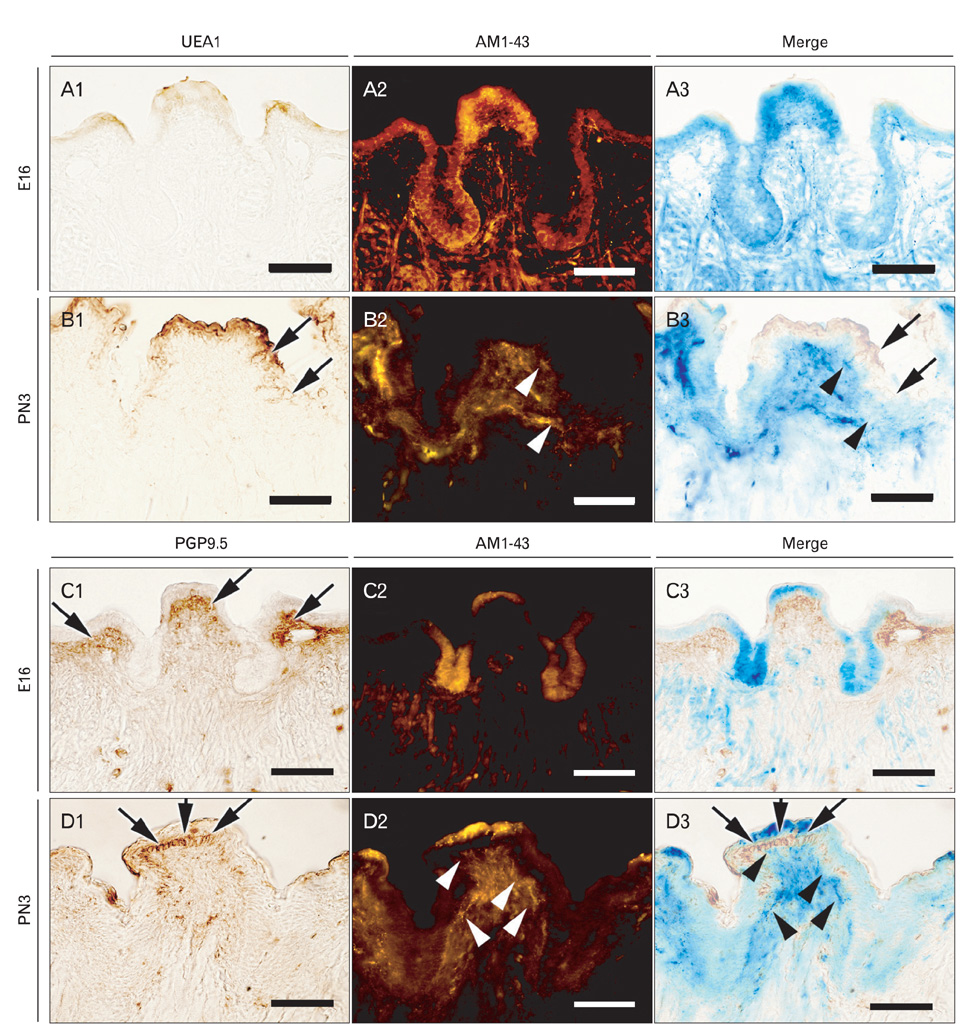
- In rodents, the circumvallate papilla (CVP), with its underlying minor salivary gland, the von Ebners' gland (VEG), is located on the dorsal surface of the posterior tongue. Detailed morphological processes to form the proper structure of CVP and VEG have not been properly elucidated. In particular, the specific localization patterns of taste buds in CVP and the branching formation of VEG have not yet been elucidated. To understand the developmental mechanisms underlying CVP and VEG formation, detailed histological observations of CVP and VEG were examined using a three-dimensional computer-aided reconstruction method with serial histological sections and pan-Cytokeratins immunostainings. In addition, to define the developmental processes in CVP and VEG formation, we examined nerve innervations and cell proliferation using microinjections of AM1-43 and immunostainings with various markers, including phosphoinositide 3-kinase, Ki-67, PGP9.5, and Ulex europaeus agglutinin 1 (UEA1). Results revealed specific morphogenesis of CVP and VEG with nerve innervations patterns, evaluated by the coincided localization patterns of AM1-43 and UEA1. Based on these morphological and immunohistochemical results, we suggest that nerve innervations and cell proliferations play important roles in the positioning of taste buds in CVP and branching morphogenesis of VEG in tongue development.
Keyword
MeSH Terms
Figure
Cited by 1 articles
-
Comparative evaluation of the ultrastructural morphology and distribution of filiform and fungiform tongue papillae in Egyptian mice, fruit bats and long-eared hedgehogs
Tahany Haggag, Elham F. Mahmoud, Zeinab A. Salem, Nermeen AbuBakr
Anat Cell Biol. 2020;53(4):493-501. doi: 10.5115/acb.20.173.
Reference
-
1. Jung HS, Akita K, Kim JY. Spacing patterns on tongue surfacegustatory papilla. Int J Dev Biol. 2004. 48:157–161.2. Spielman AI, D'Abundo S, Field RB, Schmale H. Protein analysis of human von Ebner saliva and a method for its collection from the foliate papillae. J Dent Res. 1993. 72:1331–1335.3. Sbarbati A, Crescimanno C, Merigo F, Benati D, Bernardi P, Bertini M, Osculati F. A brief survey of the modifications in sensory-secretory organs of the neonatal rat tongue. Biol Neonate. 2001. 80:1–6.4. Kim JY, Lee MJ, Cho KW, Lee JM, Kim YJ, Jung HI, Cho JY, Cho SW, Jung HS. Shh and ROCK1 modulate the dynamic epithelial morphogenesis in circumvallate papilla development. Dev Biol. 2009. 325:273–280.5. Fujimoto S, Murray RG. Fine structure of degeneration and regeneration in denervated rabbit vallate taste buds. Anat Rec. 1970. 168:383–413.6. Field RB, Dromy R, Hand AR. Regulation of secretion of enzymes from von Ebner's gland of rat tongue. J Dent Res. 1987. 66:586–587.7. Sbarbati A, Crescimanno C, Osculati F. The anatomy and functional role of the circumvallate papilla/von Ebner gland complex. Med Hypotheses. 1999. 53:40–44.8. Sbarbati A, Merigo F, Bernardi P, Crescimanno C, Benati D, Osculati F. Ganglion cells and topographically related nerves in the vallate papilla/von Ebner gland complex. J Histochem Cytochem. 2002. 50:709–718.9. Jitpukdeebodintra S, Chai Y, Snead ML. Developmental patterning of the circumvallate papilla. Int J Dev Biol. 2002. 46:755–763.10. Mbiene JP, Roberts JD. Distribution of keratin 8-containing cell clusters in mouse embryonic tongue: evidence for a prepattern for taste bud development. J Comp Neurol. 2003. 457:111–122.11. Taniguchi R, Shi L, Honma S, Fujii M, Ueda K, El-Sharaby A, Wakisaka S. Localization of Ulex europaeus agglutinin-I (UEA-I) in the developing gustatory epithelium of the rat. Arch Histol Cytol. 2004. 67:187–193.12. Lee MJ, Kim JY, Lee SI, Sasaki H, Lunny DP, Lane EB, Jung HS. Association of Shh and Ptc with keratin localization in the initiation of the formation of circumvallate papilla and von Ebner's gland. Cell Tissue Res. 2006. 325:253–261.13. Seta Y, Kataoka S, Toyono T, Toyoshima K. Immunohistochemical localization of aromatic L-amino acid decarboxylase in mouse taste buds and developing taste papillae. Histochem Cell Biol. 2007. 127:415–422.14. Fukami H, Bradley RM. Biophysical and morphological properties of parasympathetic neurons controlling the parotid and von Ebner salivary glands in rats. J Neurophysiol. 2005. 93:678–686.15. Takeda M, Suzuki Y, Obara N, Uchida N, Kawakoshi K. Expression of glial cell line-derived neurotrophic factor (GDNF) and GDNF family receptor alpha1 in mouse taste bud cells after denervation. Anat Sci Int. 2005. 80:105–110.16. Suwabe T, Fukami H, Bradley RM. Synaptic responses of neurons controlling the parotid and von Ebner salivary glands in rats to stimulation of the solitary nucleus and tract. J Neurophysiol. 2008. 99:1267–1273.17. Cheng SJ, Huang CF, Chen YC, Lee JJ, Chang HH, Chen HM, Chiang ML, Kuo MY, Kok SH, Tseng CY. Ultrastructural changes of posterior lingual glands after hypoglossal denervation in hamsters. J Anat. 2009. 214:163–170.18. Kim M, Chiego DJ Jr, Bradley RM. Morphology of parasympathetic neurons innervating rat lingual salivary glands. Auton Neurosci. 2004. 111:27–36.19. Shiraishi M, Doi Y, Kayashima K, Fujimoto S. Antioxidant enzyme immunoreactivity in rat von Ebner gland after nickel treatment. Med Mol Morphol. 2008. 41:44–52.20. Stickrod G. In utero injection of rat fetuses. Physiol Behav. 1981. 27:557–558.21. Itah R, Gitelman I, Tal J, Davis C. Viral inoculation of mouse embryos in utero. J Virol Methods. 2004. 120:1–8.22. Nishikawa S. Histochemistry of nerve fibres double labelled with anti-TRPV2 antibodies and sensory nerve marker AM1-43 in the dental pulp of rat molars. Arch Oral Biol. 2008. 53:859–864.23. Wakisaka S. Lectin histochemistry of taste buds in the circumvallate papilla of the rat. Chem Senses. 2005. 30:Suppl 1. i46–i47.24. Meyers JR, MacDonald RB, Duggan A, Lenzi D, Standaert DG, Corwin JT, Corey DP. Lighting up the senses: FM1-43 loading of sensory cells through nonselective ion channels. J Neurosci. 2003. 23:4054–4065.25. Kim JN, Koh KS, Lee E, Park SC, Song WC. The morphology of the rat vibrissal follicle-sinus complex revealed by three-dimensional computer-aided reconstruction. Cells Tissues Organs. 2011. 193:207–214.26. Freem LJ, Escot S, Tannahill D, Druckenbrod NR, Thapar N, Burns AJ. The intrinsic innervation of the lung is derived from neural crest cells as shown by optical projection tomography in Wnt1-Cre;YFP reporter mice. J Anat. 2010. 217:651–664.27. Mailleux AA, Tefft D, Ndiaye D, Itoh N, Thiery JP, Warburton D, Bellusci S. Evidence that SPROUTY2 functions as an inhibitor of mouse embryonic lung growth and morphogenesis. Mech Dev. 2001. 102:81–94.28. Cho KW, Cho SW, Lee JM, Lee MJ, Gang HS, Jung HS. Ex pression of phosphorylated forms of ERK, MEK, PTEN and PI3K in mouse oral development. Gene Expr Patterns. 2008. 8:284–290.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Differential Expression of Taste Receptors in Tongue Papillae of DBA Mouse
- Osteocalcin Expression and Mineralization in Developing Tooth of Xenopus laevis
- Prelectin Histochemical Study on Glycoconjugates of Rat Lingual Salivary Glands during the Postnatal Development
- The Development of NKT Cells in Thymus is Defective in CD45 Knockout Mice
- The Double Papilla of Vater