J Korean Soc Radiol.
2011 Aug;65(2):181-189. 10.3348/jksr.2011.65.2.181.
Imaging and Clinical Features of Thyroid Cancer in Children and Adolescents
- Affiliations
-
- 1Department of Radiology, Soonchunhyang University College of Medicine, Bucheon Hospital, Bucheon, Korea. hshong@schbc.ac.kr
- 2Department of Laboratory Medicine and Genetics, Soonchunhyang University College of Medicine, Bucheon Hospital, Bucheon, Korea.
- 3Department of Pathology, Soonchunhyang University College of Medicine, Bucheon Hospital, Bucheon, Korea.
- KMID: 1443483
- DOI: http://doi.org/10.3348/jksr.2011.65.2.181
Abstract
- PURPOSE
To evaluate clinical and imaging features of pediatric thyroid cancer, including BRAFV600E mutation status in papillary thyroid cancer (PTC).
MATERIALS AND METHODS
We evaluated clinical findings including BRAF(V600E) status, ultrasound (US), and CT features of 13 pediatric patients with thyroid cancer. US findings were retrospectively analyzed for location, presence of a nodule, echotexture, echogenicity, calcifications, margin, shape, intranodular vascularity and abnormal lymph nodes. CT characteristics of the lesions, including attenuation, calcification, and measured degree of enhancement, were assessed.
RESULTS
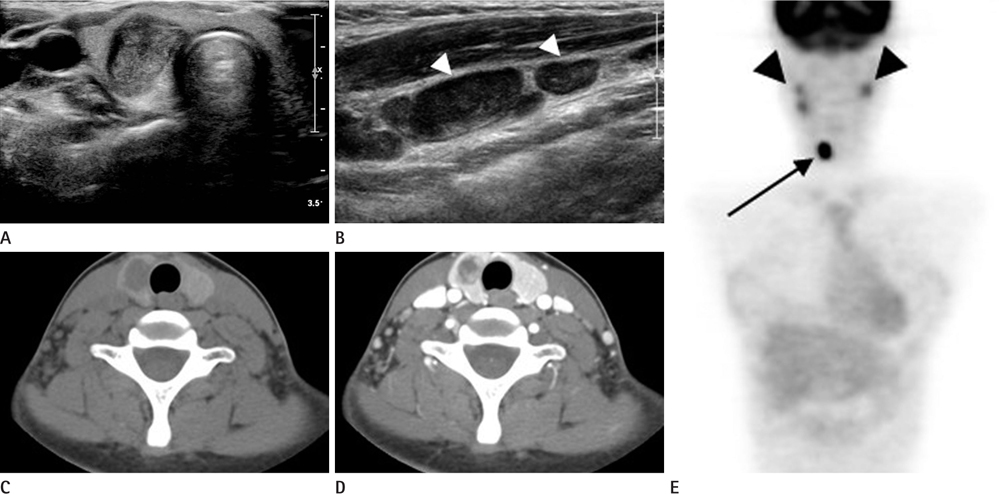
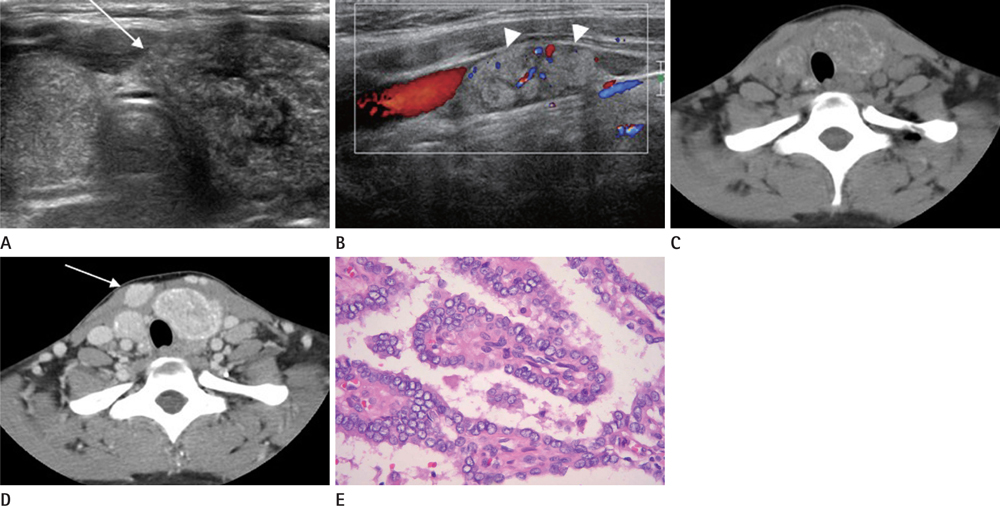
The patients included three boys and ten girls with a mean age of 15.5 years (range 6-18 years). No patient was exposed to radiation. Palpable neck mass was the most common presentation. Eleven of 13 patients (84.6%) were diagnosed with PTC, and two (15.4%) had follicular thyroid cancer (FTC). Nine of 13 (69.2%) had high T-staging. BRAF(V600E) mutations were detected in 30.0% of PTC patients. A diffusely enlarged thyroid with calcifications (n = 2) or nodules (n = 7) was detected on US. All PTC nodules showed malignant US findings and one FTC displayed on indeterminate nodule. Nodules generally showed low attenuation on enhanced CT (n = 11/12).
CONCLUSION
US demonstrated enlarged glands with calcifications or nodules. Diffusely enlarged thyroids with microcalcifications should be evaluated using fine-needle aspiration. A low attenuation nodule was a common finding on enhanced CT.
MeSH Terms
Figure
Reference
-
1. Khurana KK, Labrador E, Izquierdo R, Mesonero CE, Pisharodi LR. The role of fine-needle aspiration biopsy in the management of thyroid nodules in children, adolescents, and young adults: a multi-institutional study. Thyroid. 1999; 9:383–386.2. Collini P, Massimino M, Leite SF, Mattavelli F, Seregni E, Zucchini N, et al. Papillary thyroid carcinoma of childhood and adolescence: a 30-year experience at the Istituto Nazionale Tumori in Milan. Pediatr Blood Cancer. 2006; 46:300–306.3. Korea Central Cancer Registry Ministry of Health and Welfare Republic of Korea. 2002 Annual report of the Korea Central Cancer Registry. Seoul: Ministry of Health and Welfare Republic of Korea;2003.4. Grigsby PW, Gal-or A, Michalski JM, Doherty GM. Childhood and adolescent thyroid carcinoma. Cancer. 2002; 95:724–729.5. Zimmerman D, Hay ID, Gough IR, Goellner JR, Ryan JJ, Grant CS, et al. Papillary thyroid carcinoma in children and adults: long-term follow-up of 1039 patients conservatively treated at one institution during three decades. Surgery. 1988; 104:1157–1166.6. Brink JS, van Heerden JA, McIver B, Salomao DR, Farley DR, Grant CS, et al. Papillary thyroid cancer with pulmonary metastases in children: long-term prognosis. Surgery. 2000; 128:881–886. discussion 886-887.7. Shapiro NL, Bhattacharyya N. Population-based outcomes for pediatric thyroid carcinoma. Laryngoscope. 2005; 115:337–340.8. Fenton CL, Lukes Y, Nicholson D, Dinauer CA, Francis GL, Tuttle RM. The ret/PTC mutations are common in sporadic papillary thyroid carcinoma of children and young adults. J Clin Endocrinol Metab. 2000; 85:1170–1175.9. Ciampi R, Nikiforov YE. RET/PTC rearrangements and BRAF mutations in thyroid tumorigenesis. Endocrinology. 2007; 148:936–941.10. Santoro M, Melillo RM, Fusco A. RET/PTC activation in papillary thyroid carcinoma: European Journal of Endocrinology Prize Lecture. Eur J Endocrinol. 2006; 155:645–653.11. Xing M. BRAF mutation in thyroid cancer. Endocr Relat Cancer. 2005; 12:245–262.12. Kim KH, Kang DW, Kim SH, Seong IO, Kang DY. Mutations of the BRAF gene in papillary thyroid carcinoma in a Korean population. Yonsei Med J. 2004; 45:818–821.13. Nikiforova MN, Kimura ET, Gandhi M, Biddinger PW, Knauf JA, Basolo F, et al. BRAF mutations in thyroid tumors are restricted to papillary carcinomas and anaplastic or poorly differentiated carcinomas arising from papillary carcinomas. J Clin Endocrinol Metab. 2003; 88:5399–5404.14. Bentley AA, Gillespie C, Malis D. Evaluation and management of a solitary thyroid nodule in a child. Otolaryngol Clin North Am. 2003; 36:117–128.15. Lyshchik A, Drozd V, Demidchik Y, Reiners C. Diagnosis of thyroid cancer in children: value of gray-scale and power doppler US. Radiology. 2005; 235:604–613.16. AJCC cancer staging Manual. 7th ed. Springer-Verlag, New York: Thyroid;2010. p. 87–96.17. Moon WJ. Imaging Diagnosis. Thyroid Study group of Head and Neck society of Korean Radiology. Thyroid gland: Imaging diagnosis and Intervention. 1st ed. Seoul: Ilchokak;2008. p. 50–183.18. Recommended normative values for thyroid volume in children aged 6-15 years. World Health Organization & International Council for Control of Iodine Deficiency Disorders. Bull World Health Organ. 1997; 75:95–97.19. Demidchik YE, Demidchik EP, Reiners C, Biko J, Mine M, Saenko VA, et al. Comprehensive clinical assessment of 740 cases of surgically treated thyroid cancer in children of Belarus. Ann Surg. 2006; 243:525–532.20. Jarzab B, Handkiewicz-Junak D, Wloch J. Juvenile differentiated thyroid carcinoma and the role of radioiodine in its treatment: a qualitative review. Endocr Relat Cancer. 2005; 12:773–803.21. Nygaard B, Nygaard T, Jensen LI, Court-Payen M, Søe-Jensen P, Nielsen KG, et al. Iohexol: effects on uptake of radioactive iodine in the thyroid and on thyroid function. Acad Radiol. 1998; 5:409–414.22. Ahn JE, Lee JH, Yi JS, Shong YK, Hong SJ, Lee DH, et al. Diagnostic accuracy of CT and ultrasonography for evaluating metastatic cervical lymph nodes in patients with thyroid cancer. World J Surg. 2008; 32:1552–1558.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Ultrasonography of Various Thyroid Diseases in Children and Adolescents: A Pictorial Essay
- Treatment Outcome in Differentiated Thyroid Cancer in Children and Adolescents
- Clinical Manifestations of Chronic Autoimmune Thyroiditis in Children and Adolescents
- Ultrasonographic imaging of papillary thyroid carcinoma variants
- The treatment of Graves' disease in children and adolescents