Korean J Radiol.
2015 Apr;16(2):419-429. 10.3348/kjr.2015.16.2.419.
Ultrasonography of Various Thyroid Diseases in Children and Adolescents: A Pictorial Essay
- Affiliations
-
- 1Department of Radiology, Bucheon Hospital, Soonchunhyang University College of Medicine, Bucheon 420-767, Korea. hshong@schmc.ac.kr
- KMID: 2070186
- DOI: http://doi.org/10.3348/kjr.2015.16.2.419
Abstract
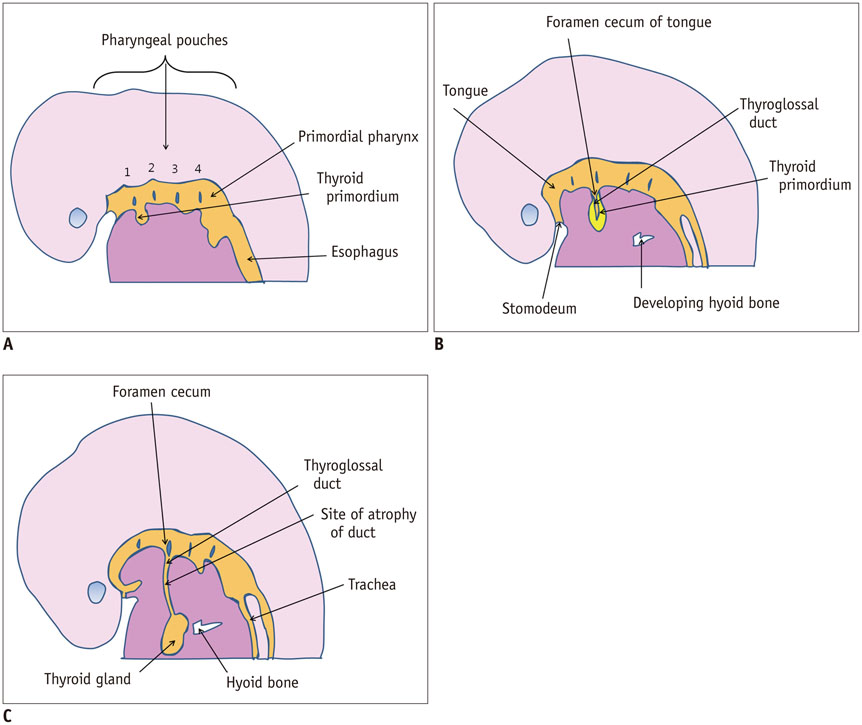
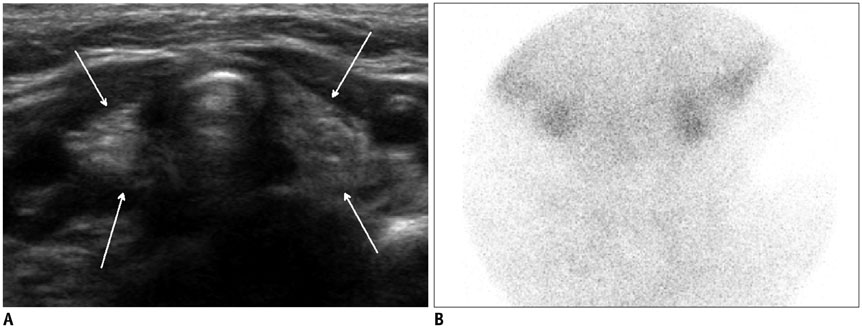
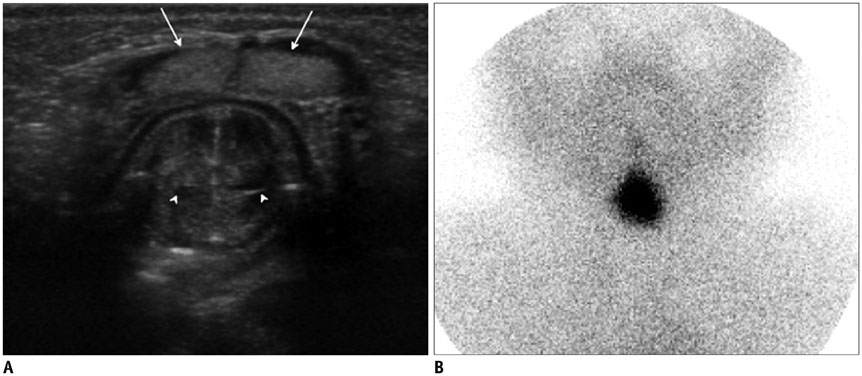
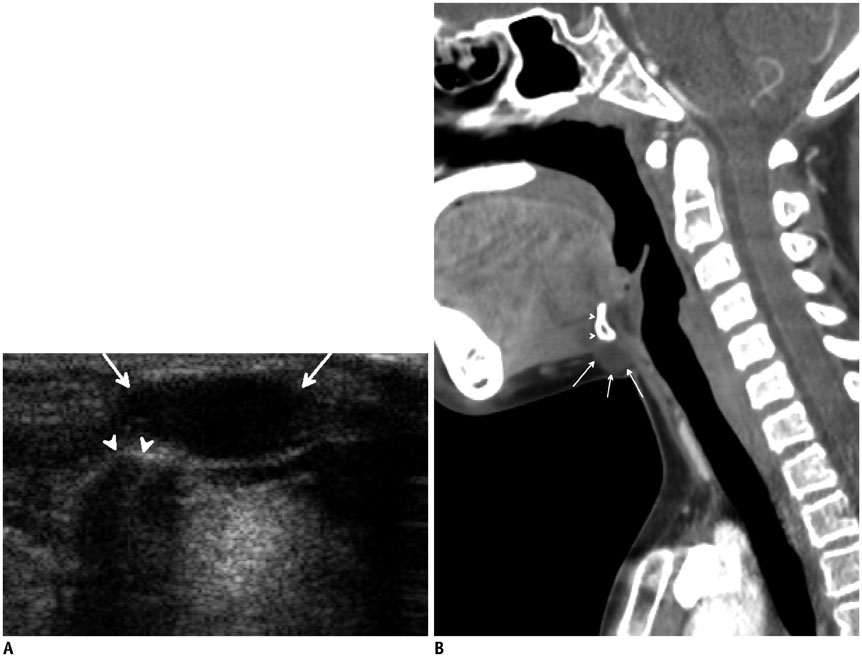
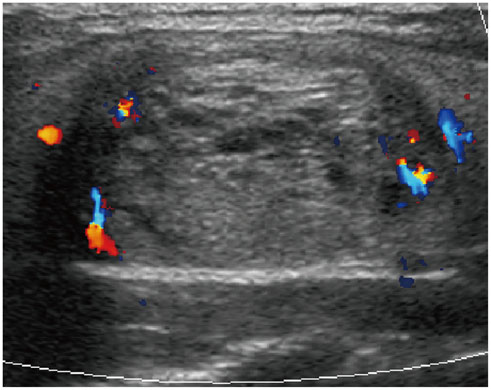
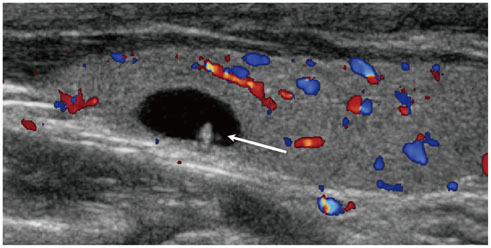
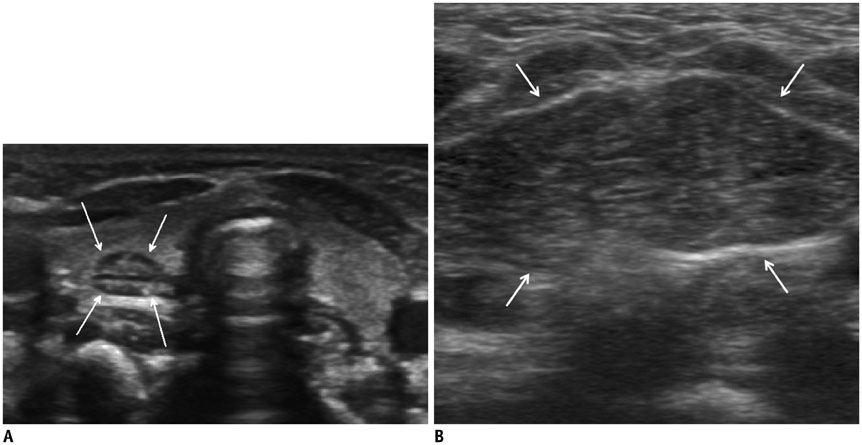
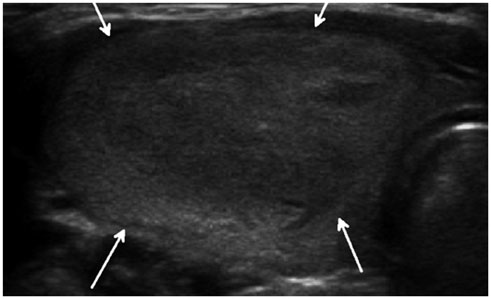
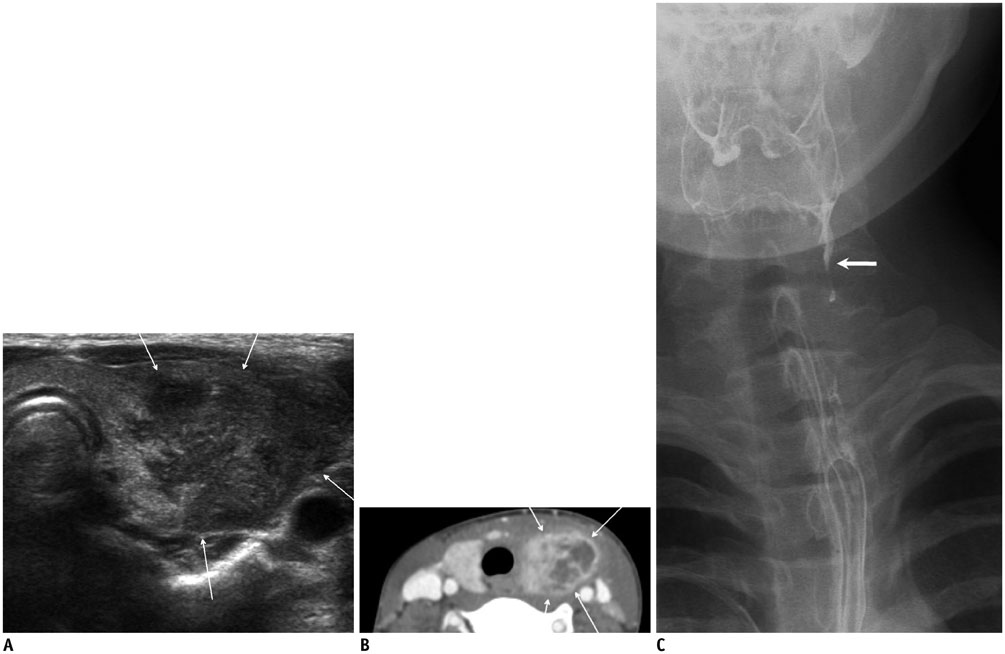
- Thyroid imaging is indicated to evaluate congenital hypothyroidism during newborn screening or in cases of a palpable thyroid mass in children and adolescents. This pictorial essay reviews the ultrasonography (US) of thyroid diseases in children and adolescents, including normal thyroid gland development, imaging features of congenital thyroid disorders (dysgenesis, [aplasia, ectopy, hypoplasia], dyshormonogenesis, transient hypothyroidism, thyroglossal duct cyst), diffuse thyroid disease (Grave's disease, Hashimoto's thyroiditis, and suppurative thyroiditis), and thyroid nodules. The primary imaging modalities for evaluating thyroid diseases are US and radionuclide scintigraphy. Additionally, US can be used to guide aspiration of detected nodules.
Keyword
MeSH Terms
-
Adolescent
Child
Congenital Hypothyroidism/diagnosis/*ultrasonography
Female
Graves Disease/diagnosis/ultrasonography
Hashimoto Disease/diagnosis/ultrasonography
Humans
Hypothyroidism/diagnosis/*ultrasonography
Infant, Newborn
Male
Thyroid Dysgenesis/diagnosis/ultrasonography
Thyroid Nodule/embryology/*ultrasonography
Thyroiditis/diagnosis/*ultrasonography
Figure
Reference
-
1. Donaldson M, Jones J. Optimising outcome in congenital hypothyroidism; current opinions on best practice in initial assessment and subsequent management. J Clin Res Pediatr Endocrinol. 2013; 5:Suppl 1. 13–22.2. Perry RJ, Maroo S, Maclennan AC, Jones JH, Donaldson MD. Combined ultrasound and isotope scanning is more informative in the diagnosis of congenital hypothyroidism than single scanning. Arch Dis Child. 2006; 91:972–976.3. Chang YW, Lee DH, Hong YH, Hong HS, Choi DL, Seo DY. Congenital hypothyroidism: analysis of discordant US and scintigraphic findings. Radiology. 2011; 258:872–879.4. Niedziela M. Pathogenesis, diagnosis and management of thyroid nodules in children. Endocr Relat Cancer. 2006; 13:427–453.5. Som PM, Smoker WR, Reidenberg JS, Bergemann AD, Hudgins PA, Laitman J. Embryology and anatomy of the neck. In : Som PM, Curtin HD, editors. Head and neck imaging. 5th ed. St. Louis, MO: Mosby;2011. p. 2117–2163.6. Zander DA, Smoker WR. Imaging of ectopic thyroid tissue and thyroglossal duct cysts. Radiographics. 2014; 34:37–50.7. Yoon HC, Kim NC, Lee DH. A cost-benefit analysis on neonatal screening of phenylketonuria and congenital hypothyroidism in Korea. Korean J Pediatr. 2005; 48:369–375.8. Lee ST, Lee DH, Kim JY, Kwon MJ, Kim JW, Hong YH, et al. Molecular screening of the TSH receptor (TSHR) and thyroid peroxidase (TPO) genes in Korean patients with nonsyndromic congenital hypothyroidism. Clin Endocrinol (Oxf). 2011; 75:715–721.9. Macchia PE. Recent advances in understanding the molecular basis of primary congenital hypothyroidism. Mol Med Today. 2000; 6:36–42.10. Korzeniewski SJ, Grigorescu V, Kleyn M, Young WI, Birbeck G, Todem D, et al. Transient hypothyroidism at 3-year follow-up among cases of congenital hypothyroidism detected by newborn screening. J Pediatr. 2013; 162:177–182.11. Mitchell ML, Hsu HW, Sahai I. Massachusetts Pediatric Endocrine Work Group. The increased incidence of congenital hypothyroidism: fact or fancy? Clin Endocrinol (Oxf). 2011; 75:806–810.12. Davy T, Daneman D, Walfish PG, Ehrlich RM. Congenital hypothyroidism. The effect of stopping treatment at 3 years of age. Am J Dis Child. 1985; 139:1028–1030.13. Jeon G, Shin JH, Hong HS, Kim JH, Hwang JH, Goo DE, et al. Sonographic measurement of normal thyroid gland in the neonates. J Korean Soc Med Ultrasound. 2001; 20:207–212.14. Ueda D. Normal volume of the thyroid gland in children. J Clin Ultrasound. 1990; 18:455–462.15. Perry RJ, Hollman AS, Wood AM, Donaldson MD. Ultrasound of the thyroid gland in the newborn: normative data. Arch Dis Child Fetal Neonatal Ed. 2002; 87:F209–F211.16. Vade A, Gottschalk ME, Yetter EM, Subbaiah P. Sonographic measurements of the neonatal thyroid gland. J Ultrasound Med. 1997; 16:395–399.17. Recommended normative values for thyroid volume in children aged 6-15 years. World Health Organization & International Council for Control of Iodine Deficiency Disorders. Bull World Health Organ. 1997; 75:95–97.18. Som PM, Smoker WR, Curtin HD, Reidenberg JS, Laitman J. Congenital lesions of the neck. In : Som PM, Curtin HD, editors. Head and neck imaging. 5th ed. St. Louis, MO: Mosby;2011. p. 2235–2285.19. Khurana KK, Labrador E, Izquierdo R, Mesonero CE, Pisharodi LR. The role of fine-needle aspiration biopsy in the management of thyroid nodules in children, adolescents, and young adults: a multi-institutional study. Thyroid. 1999; 9:383–386.20. Avula S, Daneman A, Navarro OM, Moineddin R, Urbach S, Daneman D. Incidental thyroid abnormalities identified on neck US for non-thyroid disorders. Pediatr Radiol. 2010; 40:1774–1780.21. Hung W, Anderson KD, Chandra RS, Kapur SP, Patterson K, Randolph JG, et al. Solitary thyroid nodules in 71 children and adolescents. J Pediatr Surg. 1992; 27:1407–1409.22. Moon WJ, Baek JH, Jung SL, Kim DW, Kim EK, Kim JY, et al. Ultrasonography and the ultrasound-based management of thyroid nodules: consensus statement and recommendations. Korean J Radiol. 2011; 12:1–14.23. Babcock DS. Thyroid disease in the pediatric patient: emphasizing imaging with sonography. Pediatr Radiol. 2006; 36:299–308. quiz 372-373.24. Chang YW, Hong HS, Choi DL. Sonography of the pediatric thyroid: a pictorial essay. J Clin Ultrasound. 2009; 37:149–157.25. Megremis S, Stiakaki E, Tritou I, Bonapart IE, Tsilimigaki A. Ectopic intrathyroidal thymus misdiagnosed as a thyroid nodule: sonographic appearance. J Clin Ultrasound. 2008; 36:443–447.26. Korea Central Cancer Registry Ministry of Health and Welfare. Republic of Korea. 2011 Annual report of the Korea. Central Cancer Registry. Seoul: Ministry of Health and Welfare Republic of Korea;2014. Accessed April 2004. Web site. http://www.ncc.re.kr/.27. Grigsby PW, Gal-or A, Michalski JM, Doherty GM. Childhood and adolescent thyroid carcinoma. Cancer. 2002; 95:724–729.28. Brink JS, van Heerden JA, McIver B, Salomao DR, Farley DR, Grant CS, et al. Papillary thyroid cancer with pulmonary metastases in children: long-term prognosis. Surgery. 2000; 128:881–886. discussion 886-887.29. Shin LK, Olcott EW, Jeffrey RB, Desser TS. Sonographic evaluation of cervical lymph nodes in papillary thyroid cancer. Ultrasound Q. 2013; 29:25–32.30. Fenton CL, Lukes Y, Nicholson D, Dinauer CA, Francis GL, Tuttle RM. The ret/PTC mutations are common in sporadic papillary thyroid carcinoma of children and young adults. J Clin Endocrinol Metab. 2000; 85:1170–1175.31. Lee KY, Hong HS, Lee EH, Yi BH, Lee HK, Lee YW, et al. Imaging and clinical features of thyroid cancer in children and adolescents. J Korean Soc Radiol. 2011; 65:181–189.32. Xing M. BRAF mutation in thyroid cancer. Endocr Relat Cancer. 2005; 12:245–262.33. Yang JY, Hong HS, Lee EH, Kim CH, Kwak JJ, Lee SW, et al. Analysis of the BRAFV600E mutation in thyroid nodules: the preoperative diagnostic role of fine-needle aspiration biopsy for patients with papillary thyroid cancer and its impact on patient care. J Korean Soc Ultrasound Med. 2011; 30:103–112.34. Grebe SK, Hay ID. Follicular thyroid cancer. Endocrinol Metab Clin North Am. 1995; 24:761–801.35. Nikiforova MN, Lynch RA, Biddinger PW, Alexander EK, Dorn GW 2nd, Tallini G, et al. RAS point mutations and PAX8-PPAR gamma rearrangement in thyroid tumors: evidence for distinct molecular pathways in thyroid follicular carcinoma. J Clin Endocrinol Metab. 2003; 88:2318–2326.36. Volante M, Rapa I, Gandhi M, Bussolati G, Giachino D, Papotti M, et al. RAS mutations are the predominant molecular alteration in poorly differentiated thyroid carcinomas and bear prognostic impact. J Clin Endocrinol Metab. 2009; 94:4735–4741.37. Pearce EN, Farwell AP, Braverman LE. Thyroiditis. N Engl J Med. 2003; 348:2646–2655.38. Yeh HC, Futterweit W, Gilbert P. Micronodulation: ultrasonographic sign of Hashimoto thyroiditis. J Ultrasound Med. 1996; 15:813–819.39. Pedersen OM, Aardal NP, Larssen TB, Varhaug JE, Myking O, Vik-Mo H. The value of ultrasonography in predicting autoimmune thyroid disease. Thyroid. 2000; 10:251–259.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Thyroid disease in children and adolescents
- Breast lesions during pregnancy and lactation: a pictorial essay
- Unusual, but important, peri- and extra-articular manifestations of rheumatoid arthritis: a pictorial essay
- Sonographic Features of Palpable Breast and Axillary Lesions in Adult Male Patients: A Pictorial Essay
- Navigated intraoperative ultrasonography for brain tumors: a pictorial essay on the technique, its utility, and its benefits in neuro-oncology