Yonsei Med J.
2012 Nov;53(6):1165-1175. 10.3349/ymj.2012.53.6.1165.
Gamma Linolenic Acid Exerts Anti-Inflammatory and Anti-Fibrotic Effects in Diabetic Nephropathy
- Affiliations
-
- 1Department of Internal Medicine, Brain Korea 21 Project for Medical Science, Yonsei University College of Medicine, Seoul, Korea. kswkidney@yuhs.ac
- KMID: 1414260
- DOI: http://doi.org/10.3349/ymj.2012.53.6.1165
Abstract
- PURPOSE
This study was undertaken to investigate the effects of gamma linolenic acid (GLA) on inflammation and extracellular matrix (ECM) synthesis in mesangial and tubular epithelial cells under diabetic conditions.
MATERIALS AND METHODS
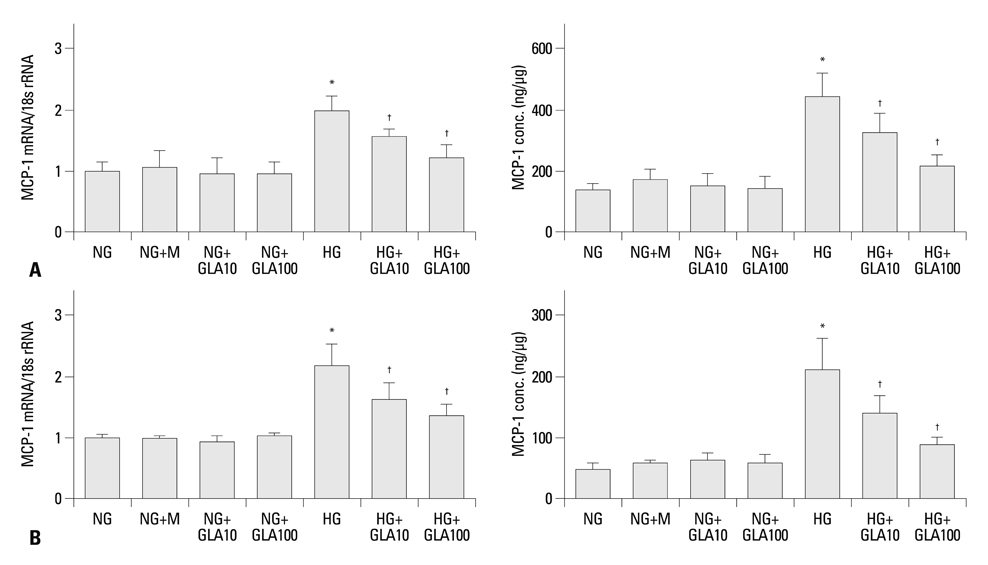
Sprague-Dawley rats were intraperitoneally injected with either a diluent [n=16, control (C)] or streptozotocin [n=16, diabetes (DM)], and eight rats each from the control and diabetic groups were treated with evening primrose oil by gavage for three months. Rat mesangial cells and NRK-52E cells were exposed to medium containing 5.6 mM glucose and 30 mM glucose (HG), with or without GLA (10 or 100 microM). Intercellular adhesion molecule-1 (ICAM-1), monocyte chemoattractant protein-1 (MCP-1), and fibronectin (FN) mRNA and protein expression levels were evaluated.
RESULTS
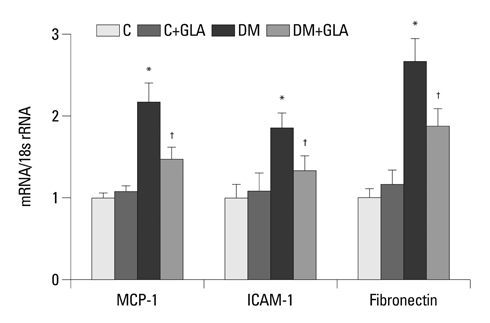
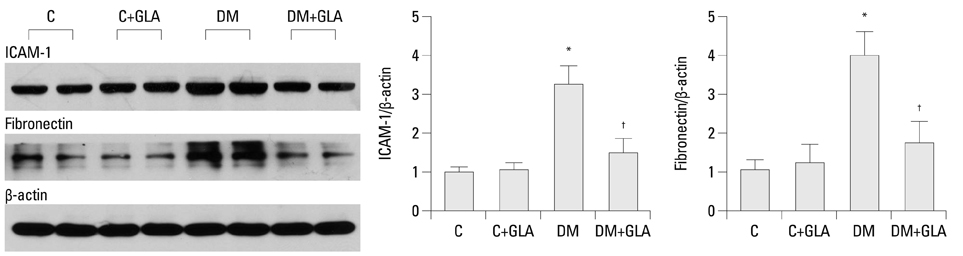
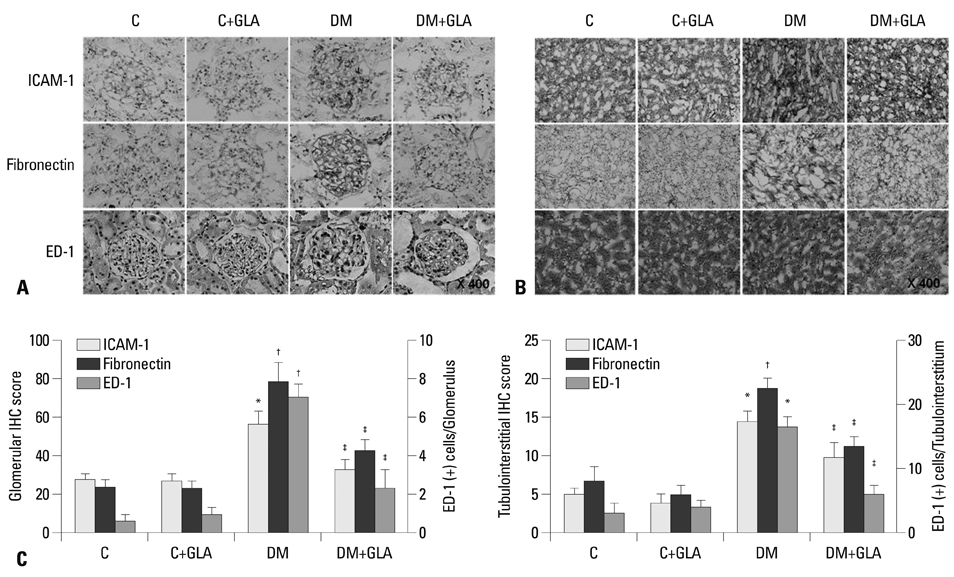
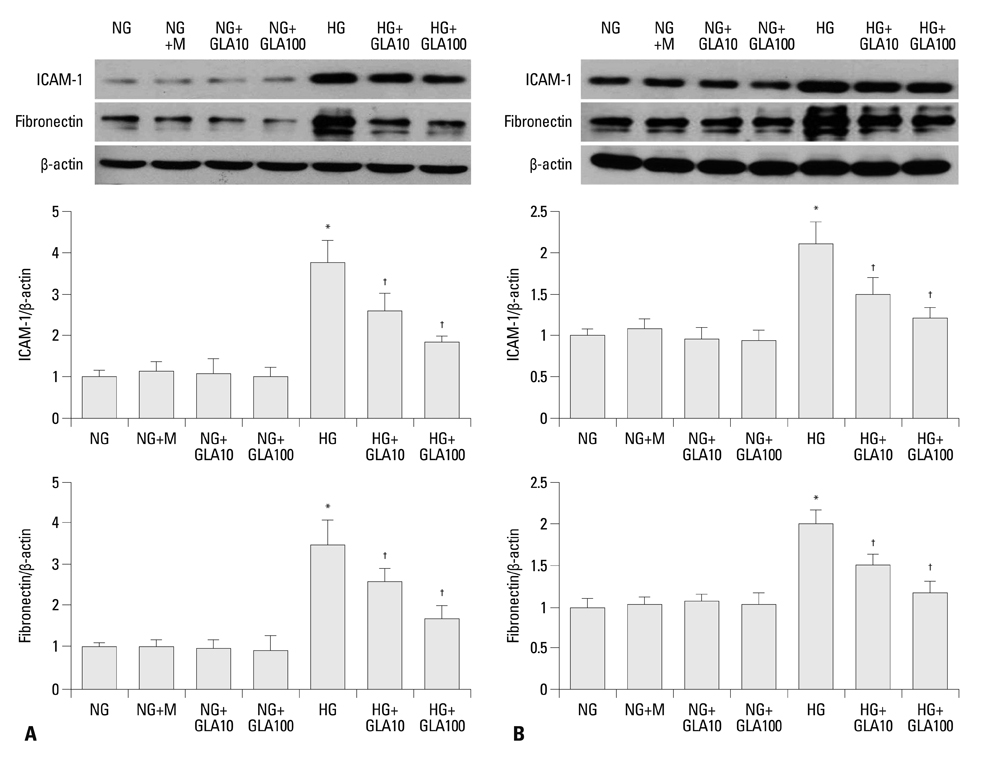
Twenty-four-hour urinary albumin excretion was significantly increased in DM compared to C rats, and GLA treatment significantly reduced albuminuria in DM rats. ICAM-1, MCP-1, FN mRNA and protein expression levels were significantly higher in DM than in C kidneys, and these increases were significantly abrogated by GLA treatment. In vitro, GLA significantly inhibited increases in MCP-1 mRNA expression and protein levels under high glucose conditions in HG-stimulated mesangial and tubular epithelial cells (p<0.05, respectively). ICAM-1 and FN expression showed a similar pattern to the expression of MCP-1.
CONCLUSION
GLA attenuates not only inflammation by inhibiting enhanced MCP-1 and ICAM-1 expression, but also ECM accumulation in diabetic nephropathy.
MeSH Terms
-
Animals
Anti-Inflammatory Agents/*therapeutic use
Blotting, Western
Chemokine CCL2/genetics/metabolism
Diabetic Nephropathies/*drug therapy/*metabolism
Enzyme-Linked Immunosorbent Assay
Fibronectins/genetics/metabolism
Intercellular Adhesion Molecule-1/genetics/metabolism
Rats
Rats, Sprague-Dawley
Real-Time Polymerase Chain Reaction
alpha-Linolenic Acid/*therapeutic use
Figure
Cited by 1 articles
-
The Effect of Evening Primrose Oil for the Prevention of Xerotic Cheilitis in Acne Patients Being Treated with Isotretinoin: A Pilot Study
Kui Young Park, Eun Jung Ko, In Su Kim, Kapsok Li, Beom Joon Kim, Seong Jun Seo, Myeung Nam Kim, Chang Kwun Hong
Ann Dermatol. 2014;26(6):706-712. doi: 10.5021/ad.2014.26.6.706.
Reference
-
1. Wolf G, Ziyadeh FN. Molecular mechanisms of diabetic renal hypertrophy. Kidney Int. 1999. 56:393–405.
Article2. Chen S, Jim B, Ziyadeh FN. Diabetic nephropathy and transforming growth factor-beta: transforming our view of glomerulosclerosis and fibrosis build-up. Semin Nephrol. 2003. 23:532–543.
Article3. Solini A, Iacobini C, Ricci C, Chiozzi P, Amadio L, Pricci F, et al. Purinergic modulation of mesangial extracellular matrix production: role in diabetic and other glomerular diseases. Kidney Int. 2005. 67:875–885.
Article4. Navarro-González JF, Mora-Fernández C. The role of inflammatory cytokines in diabetic nephropathy. J Am Soc Nephrol. 2008. 19:433–442.
Article5. Furuta T, Saito T, Ootaka T, Soma J, Obara K, Abe K, et al. The role of macrophages in diabetic glomerulosclerosis. Am J Kidney Dis. 1993. 21:480–485.
Article6. Young BA, Johnson RJ, Alpers CE, Eng E, Gordon K, Floege J, et al. Cellular events in the evolution of experimental diabetic nephropathy. Kidney Int. 1995. 47:935–944.
Article7. Banba N, Nakamura T, Matsumura M, Kuroda H, Hattori Y, Kasai K. Possible relationship of monocyte chemoattractant protein-1 with diabetic nephropathy. Kidney Int. 2000. 58:684–690.
Article8. Sugimoto H, Shikata K, Hirata K, Akiyama K, Matsuda M, Kushiro M, et al. Increased expression of intercellular adhesion molecule-1 (ICAM-1) in diabetic rat glomeruli: glomerular hyperfiltration is a potential mechanism of ICAM-1 upregulation. Diabetes. 1997. 46:2075–2081.
Article9. Utimura R, Fujihara CK, Mattar AL, Malheiros DM, Noronha IL, Zatz R. Mycophenolate mofetil prevents the development of glomerular injury in experimental diabetes. Kidney Int. 2003. 63:209–216.
Article10. Nasrallah R, Robertson SJ, Hébert RL. Chronic COX inhibition reduces diabetes-induced hyperfiltration, proteinuria, and renal pathological markers in 36-week B6-Ins2 (Akita) mice. Am J Nephrol. 2009. 30:346–353.
Article11. Benatti P, Peluso G, Nicolai R, Calvani M. Polyunsaturated fatty acids: biochemical, nutritional and epigenetic properties. J Am Coll Nutr. 2004. 23:281–302.
Article12. Buist PH. Fatty acid desaturases: selecting the dehydrogenation channel. Nat Prod Rep. 2004. 21:249–262.
Article13. Fan YY, Chapkin RS. Importance of dietary gamma-linolenic acid in human health and nutrition. J Nutr. 1998. 128:1411–1414.14. Ingram AJ, Parbtani A, Clark WF, Spanner E, Huff MW, Philbrick DJ, et al. The Nutrition & Kidney Disease Research Group. Dietary alteration of dihomogamma-linolenic acid/arachidonic acid ratio in a rat 5/6-renal-ablation model. J Am Soc Nephrol. 1996. 7:1024–1031.
Article15. Zurier RB, Rossetti RG, Jacobson EW, DeMarco DM, Liu NY, Temming JE, et al. gamma-Linolenic acid treatment of rheumatoid arthritis. A randomized, placebo-controlled trial. Arthritis Rheum. 1996. 39:1808–1817.
Article16. Keen H, Payan J, Allawi J, Walker J, Jamal GA, Weir AI, et al. The gamma-Linolenic Acid Multicenter Trial Group. Treatment of diabetic neuropathy with gamma-linolenic acid. Diabetes Care. 1993. 16:8–15.17. Li JJ, Lee SH, Kim DK, Jin R, Jung DS, Kwak SJ, et al. Colchicine attenuates inflammatory cell infiltration and extracellular matrix accumulation in diabetic nephropathy. Am J Physiol Renal Physiol. 2009. 297:F200–F209.
Article18. Huang GT, Eckmann L, Savidge TC, Kagnoff MF. Infection of human intestinal epithelial cells with invasive bacteria upregulates apical intercellular adhesion molecule-1 (ICAM)-1) expression and neutrophil adhesion. J Clin Invest. 1996. 98:572–583.
Article19. Rovin BH, Yoshiumura T, Tan L. Cytokine-induced production of monocyte chemoattractant protein-1 by cultured human mesangial cells. J Immunol. 1992. 148:2148–2153.20. Chiarelli F, Cipollone F, Mohn A, Marini M, Iezzi A, Fazia M, et al. Circulating monocyte chemoattractant protein-1 and early development of nephropathy in type 1 diabetes. Diabetes Care. 2002. 25:1829–1834.
Article21. Coimbra TM, Janssen U, Gröne HJ, Ostendorf T, Kunter U, Schmidt H, et al. Early events leading to renal injury in obese Zucker (fatty) rats with type II diabetes. Kidney Int. 2000. 57:167–182.
Article22. Yeo ES, Hwang JY, Park JE, Choi YJ, Huh KB, Kim WY. Tumor necrosis factor (TNF-alpha) and C-reactive protein (CRP) are positively associated with the risk of chronic kidney disease in patients with type 2 diabetes. Yonsei Med J. 2010. 51:519–525.
Article23. Kikuchi Y, Imakiire T, Yamada M, Saigusa T, Hyodo T, Hyodo N, et al. Mizoribine reduces renal injury and macrophage infiltration in non-insulin-dependent diabetic rats. Nephrol Dial Transplant. 2005. 20:1573–1581.
Article24. Chow FY, Nikolic-Paterson DJ, Ozols E, Atkins RC, Tesch GH. Intercellular adhesion molecule-1 deficiency is protective against nephropathy in type 2 diabetic db/db mice. J Am Soc Nephrol. 2005. 16:1711–1722.
Article25. Chow FY, Nikolic-Paterson DJ, Ma FY, Ozols E, Rollins BJ, Tesch GH. Monocyte chemoattractant protein-1-induced tissue inflammation is critical for the development of renal injury but not type 2 diabetes in obese db/db mice. Diabetologia. 2007. 50:471–480.
Article26. Kim DK, Nam BY, Li JJ, Park JT, Lee SH, Kim DH, et al. Translationally controlled tumour protein is associated with podocyte hypertrophy in a mouse model of type 1 diabetes. Diabetologia. 2012. 55:1205–1217.
Article27. Nolin L, Courteau M. Management of IgA nephropathy: evidence-based recommendations. Kidney Int Suppl. 1999. 70:S56–S62.
Article28. Fassett RG, Gobe GC, Peake JM, Coombes JS. Omega-3 polyunsaturated fatty acids in the treatment of kidney disease. Am J Kidney Dis. 2010. 56:728–742.
Article29. Das UN. Essential fatty acid metabolism in patients with essential hypertension, diabetes mellitus and coronary heart disease. Prostaglandins Leukot Essent Fatty Acids. 1995. 52:387–391.
Article30. Hounsom L, Horrobin DF, Tritschler H, Corder R, Tomlinson DR. A lipoic acid-gamma linolenic acid conjugate is effective against multiple indices of experimental diabetic neuropathy. Diabetologia. 1998. 41:839–843.
Article31. Forman BM, Chen J, Evans RM. Hypolipidemic drugs, polyunsaturated fatty acids, and eicosanoids are ligands for peroxisome proliferator-activated receptors alpha and delta. Proc Natl Acad Sci U S A. 1997. 94:4312–4317.
Article32. Pham H, Banerjee T, Nalbandian GM, Ziboh VA. Activation of peroxisome proliferator-activated receptor (PPAR)-gamma by 15S-hydroxyeicosatrienoic acid parallels growth suppression of androgen-dependent prostatic adenocarcinoma cells. Cancer Lett. 2003. 189:17–25.
Article33. Daynes RA, Jones DC. Emerging roles of PPARs in inflammation and immunity. Nat Rev Immunol. 2002. 2:748–759.
Article34. Zhang Y, Guan Y. PPAR-gamma agonists and diabetic nephropathy. Curr Diab Rep. 2005. 5:470–475.35. Watanabe M, Nakashima H, Mochizuki S, Abe Y, Ishimura A, Ito K, et al. Amelioration of diabetic nephropathy in OLETF rats by prostaglandin I(2) analog, beraprost sodium. Am J Nephrol. 2009. 30:1–11.
Article36. Takata S, Matsubara M, Allen PG, Janmey PA, Serhan CN, Brady HR. Remodeling of neutrophil phospholipids with 15(S)-hydroxyeicosatetraenoic acid inhibits leukotriene B4-induced neutrophil migration across endothelium. J Clin Invest. 1994. 93:499–508.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Combination Treatment with Retinoid and Peroxisome Proliferator-Activated Receptors (PPAR)-gamma Agonist on Streptozotocin-Induced Diabetic Nephropathy
- The Anti-inflammatory Effect of Retinoid on Streptozotocin-induced Diabetic Nephropathy
- Effects of Gamma-Linolenic Acid for the Treatment of Acne Vulgaris Treated with Isotretinoin
- Isomer specificity of conjugated linoleic acid (CLA): 9E,11E-CLA
- The Effect of Isoflavone and Gamma-linolenic Acid Supplementation on Serum Lipids and Menopausal Symptoms in Postmenopausal Women