Korean J Radiol.
2012 Dec;13(6):702-710. 10.3348/kjr.2012.13.6.702.
Dual-Energy CT in Patients Treated with Anti-Angiogenic Agents for Non-Small Cell Lung Cancer: New Method of Monitoring Tumor Response?
- Affiliations
-
- 1Department of Radiology and Center for Imaging Science, Samsung Medical Center, Sungkyunkwan University School of Medicine, Seoul 135-710, Korea. hoyunlee96@gmail.com
- 2Division of Hemato-Oncology, Department of Internal Medicine, Samsung Medical Center, Sungkyunkwan University School of Medicine, Seoul 135-710, Korea.
- 3Department of Radiology and Research Institute of Radiology, Asan Medical Center, University of Ulsan College of Medicine, Seoul 138-736, Korea.
- KMID: 1397500
- DOI: http://doi.org/10.3348/kjr.2012.13.6.702
Abstract
OBJECTIVE
To evaluate tumor responses in patients treated with anti-angiogenic agents for non-small cell lung cancer (NSCLC) by assessing intratumoral changes using a dual-energy CT (DECT) (based on Choi's criteria) and to compare it to traditional Response Evaluation Criteria in Solid Tumors (RECIST) criteria.
MATERIALS AND METHODS
Ten NSCLC patients treated with bevacizumab underwent DECT. Tumor responses to anti-angiogenic therapy were assessed and compared with the baseline CT results using both RECIST (size changes only) and Choi's criteria (reflecting net tumor enhancement). Kappa statistics was used to evaluate agreements between tumor responses assessed by RECIST and Choi's criteria.
RESULTS
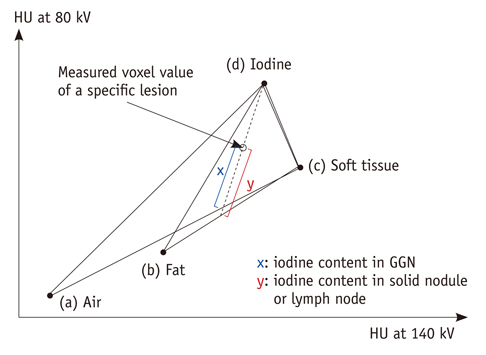
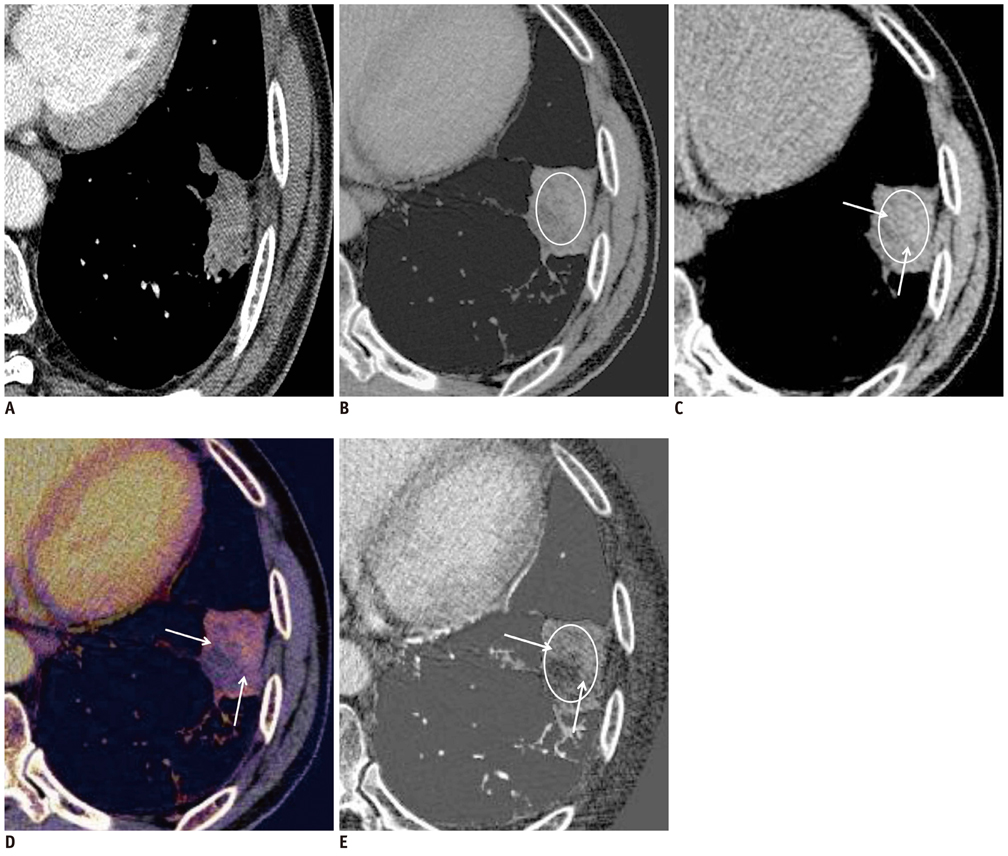
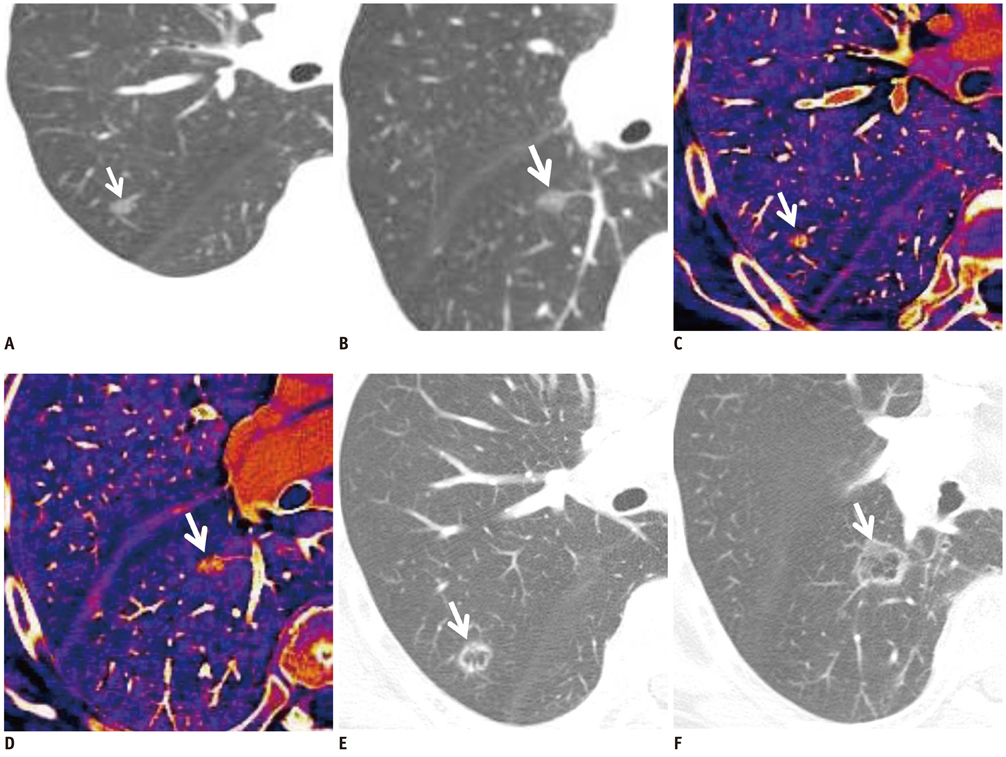
The weighted kappa value for the comparison of tumor responses between the RECIST and Choi's criteria was 0.72. Of 31 target lesions (21 solid nodules, 8 lymph nodes, and two ground-glass opacity nodules [GGNs]), five lesions (16%) showed discordant responses between RECIST and Choi's criteria. Iodine-enhanced images allowed for a distinction between tumor enhancement and hemorrhagic response (detected in 14% [4 of 29, excluding GGNs] of target lesions on virtual nonenhanced images).
CONCLUSION
DECT may serve as a useful tool for response evaluation after anti-angiogenic treatment in NSCLC patients by providing information on the net enhancement of target lesions without obtaining non-enhanced images.
Keyword
MeSH Terms
Figure
Reference
-
1. Eisenhauer EA, Therasse P, Bogaerts J, Schwartz LH, Sargent D, Ford R, et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur J Cancer. 2009. 45:228–247.2. Pirker R, Filipits M. Targeted therapies in lung cancer. Curr Pharm Des. 2009. 15:188–206.3. Bertino EM, Otterson GA. Benefits and limitations of antiangiogenic agents in patients with non-small cell lung cancer. Lung Cancer. 2010. 70:233–246.4. Lee HY, Lee KS, Ahn MJ, Hwang HS, Lee JW, Park K, et al. New CT response criteria in non-small cell lung cancer: proposal and application in EGFR tyrosine kinase inhibitor therapy. Lung Cancer. 2011. 73:63–69.5. Johnson DH, Fehrenbacher L, Novotny WF, Herbst RS, Nemunaitis JJ, Jablons DM, et al. Randomized phase II trial comparing bevacizumab plus carboplatin and paclitaxel with carboplatin and paclitaxel alone in previously untreated locally advanced or metastatic non-small-cell lung cancer. J Clin Oncol. 2004. 22:2184–2191.6. Sandler AB, Schiller JH, Gray R, Dimery I, Brahmer J, Samant M, et al. Retrospective evaluation of the clinical and radiographic risk factors associated with severe pulmonary hemorrhage in first-line advanced, unresectable non-small-cell lung cancer treated with Carboplatin and Paclitaxel plus bevacizumab. J Clin Oncol. 2009. 27:1405–1412.7. Choi H, Charnsangavej C, Faria SC, Macapinlac HA, Burgess MA, Patel SR, et al. Correlation of computed tomography and positron emission tomography in patients with metastatic gastrointestinal stromal tumor treated at a single institution with imatinib mesylate: proposal of new computed tomography response criteria. J Clin Oncol. 2007. 25:1753–1759.8. Coursey CA, Nelson RC, Boll DT, Paulson EK, Ho LM, Neville AM, et al. Dual-energy multidetector CT: how does it work, what can it tell us, and when can we use it in abdominopelvic imaging? Radiographics. 2010. 30:1037–1055.9. Kang MJ, Park CM, Lee CH, Goo JM, Lee HJ. Dual-energy CT: clinical applications in various pulmonary diseases. Radiographics. 2010. 30:685–698.10. Johnson TR, Krauss B, Sedlmair M, Grasruck M, Bruder H, Morhard D, et al. Material differentiation by dual energy CT: initial experience. Eur Radiol. 2007. 17:1510–1517.11. Chae EJ, Song JW, Seo JB, Krauss B, Jang YM, Song KS. Clinical utility of dual-energy CT in the evaluation of solitary pulmonary nodules: initial experience. Radiology. 2008. 249:671–681.12. Schmid-Bindert G, Henzler T, Chu TQ, Meyer M, Nance JW Jr, Schoepf UJ, et al. Functional imaging of lung cancer using dual energy CT: how does iodine related attenuation correlate with standardized uptake value of 18FDG-PET-CT? Eur Radiol. 2012. 22:93–103.13. Gupta R, Phan CM, Leidecker C, Brady TJ, Hirsch JA, Nogueira RG, et al. Evaluation of dual-energy CT for differentiating intracerebral hemorrhage from iodinated contrast material staining. Radiology. 2010. 257:205–211.14. Ling D, Korobkin M, Silverman PM, Dunnick NR. CT demonstration of bilateral adrenal hemorrhage. AJR Am J Roentgenol. 1983. 141:307–308.15. Crabb SJ, Patsios D, Sauerbrei E, Ellis PM, Arnold A, Goss G, et al. Tumor cavitation: impact on objective response evaluation in trials of angiogenesis inhibitors in non-small-cell lung cancer. J Clin Oncol. 2009. 27:404–410.16. Lee HY, Lee KS, Hwang HS, Lee JW, Ahn MJ, Park K, et al. Molecularly targeted therapy using bevacizumab for non-small cell lung cancer: a pilot study for the new CT response criteria. Korean J Radiol. 2010. 11:618–626.17. Graser A, Johnson TR, Hecht EM, Becker CR, Leidecker C, Staehler M, et al. Dual-energy CT in patients suspected of having renal masses: can virtual nonenhanced images replace true nonenhanced images? Radiology. 2009. 252:433–440.18. Barrett T, Bowden DJ, Shaida N, Godfrey EM, Taylor A, Lomas DJ, et al. Virtual unenhanced second generation dual-source CT of the liver: is it time to discard the conventional unenhanced phase? Eur J Radiol. 2012. 81:1438–1445.19. Hansell DM, Bankier AA, MacMahon H, McLoud TC, Müller NL, Remy J. Fleischner Society: glossary of terms for thoracic imaging. Radiology. 2008. 246:697–722.20. Schenzle JC, Sommer WH, Neumaier K, Michalski G, Lechel U, Nikolaou K, et al. Dual energy CT of the chest: how about the dose? Invest Radiol. 2010. 45:347–353.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- The Prognostic Role of Vascular Endothelial Growth Factor (VEGF) Expression and Angiogenesis in Curatively Resected Non-Small Cell Lung Cancer
- Prognostic Value of Vascular Endothelial Growth Factor(VEGF) in Resected Non-Small Cell Lung Cancer
- Clinical Significant of S-Phase Fraction in Small Lung Cancer
- Radiation Effect on Airway Obstruction from Lung Cancer
- Radiation Results and Survival Rate of Small Cell Lung Cancer