J Vet Sci.
2012 Sep;13(3):253-259. 10.4142/jvs.2012.13.3.253.
Hsp70 and HSF-1 expression is altered in the tissues of pigs transported for various periods of times
- Affiliations
-
- 1College of Veterinary Medicine, Nanjing Agricultural University, Nanjing 210095, China. b_endong@njau.edu.cn
- 2College of Animal Science and Technology, Jinling Institute of Technology, Nanjing 210038, China.
- 3Institute for Animal Hygiene, Animal Welfare and Farm Animal Behaviour, University of Veterinary Medicine Hannover, Foundation, 30559 Hannover, Germany.
- KMID: 1389764
- DOI: http://doi.org/10.4142/jvs.2012.13.3.253
Abstract
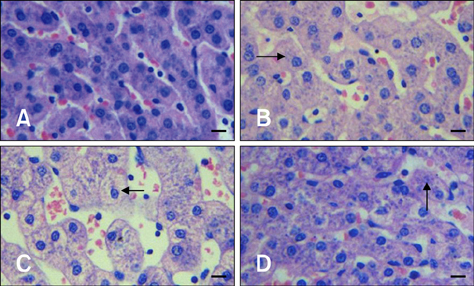
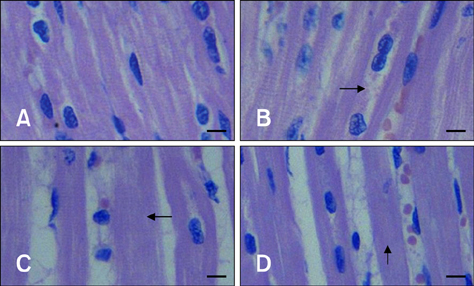
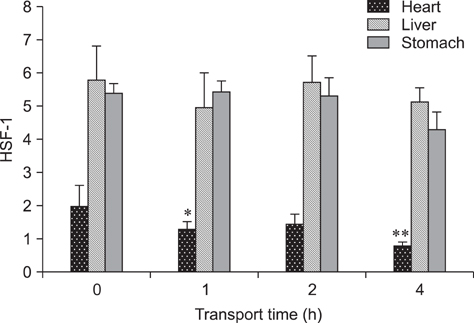
- The aim of this study was to assess changes of Hsp70 and HSF-1 protein and mRNA expression in stress-sensitive organs of pigs during transportation for various periods of time. Twenty pigs were randomly divided into four groups (0 h, 1 h, 2 h, and 4 h of transportation). A significant increased activity of AST and CK was observed after 1 h and 2 h of transportation. Histopathological changes in the heart, liver, and stomach indicated that these organs sustained different degrees of injury. Hsp70 protein expression in the heart and liver of transported pigs did not change significantly while it increased significantly (p < 0.05) in the stomach. Hsp70 mRNA levels decreased significantly (p < 0.05) in the heart after 4 h of transportation. However, mRNA expression increased significantly in the liver after 1 (p < 0.05) and 4 h (p < 0.01) of transportation, and increased significantly in the stomach of the transported pigs after 1, 4 (p < 0.01), and 2 h (p < 0.05). HSF-1 levels were reduced at 1 and 4 h (p < 0.05) only in the hearts of transported pigs. These results indicate that Hsp70 mediates distinct stress-related functions in different tissues during transportation.
Keyword
MeSH Terms
-
Animals
Creatine Kinase/blood
DNA-Binding Proteins/*metabolism
Enzyme-Linked Immunosorbent Assay/veterinary
HSP70 Heat-Shock Proteins/*metabolism
Liver/*metabolism
Myocardium/*metabolism
RNA, Messenger/metabolism
Random Allocation
Real-Time Polymerase Chain Reaction/veterinary
Stomach/*metabolism
Stress, Physiological
Swine/blood/*metabolism
Time Factors
Transaminases/blood
Transcription Factors/*metabolism
*Transportation
Figure
Reference
-
1. Abravaya K, Myers MP, Murphy SP, Morimoto RI. The human heat shock protein hsp70 interacts with HSF, the transcription factor that regulates heat shock gene expression. Genes Dev. 1992. 6:1153–1164.
Article2. Adeola O, Ball RO. Hypothalamic neurotransmitter concentrations and meat quality in stressed pigs offered excess dietary tryptophan and tyrosine. J Anim Sci. 1992. 70:1888–1894.
Article3. Banerji SS, Berg L, Morimoto RI. Transcription and post-transcriptional regulation of avian HSP70 gene expression. J Biol Chem. 1986. 261:15740–15745.
Article4. Bao E, Sultan KR, Nowak B, Hartung J. Expression and distribution of heat shock proteins in the heart of transported pigs. Cell Stress Chaperones. 2008. 13:459–466.
Article5. Bao ED, Sultan KR, Nowak B, Hartung J. Localization and expression of heat shock proteins in liver of transport stressed pigs. Zhongguo Nong Ye Ke Xue. 2002. 35:1130–1133.6. Chen G, Gharib TG, Huang CC, Taylor JMG, Misek DE, Kardia SLR, Giordano TJ, Iannettoni MD, Orringer MB, Hanash SM, Beer DG. Discordant protein and mRNA expression in lung adenocarcinomas. Mol Cell Proteomics. 2002. 1:304–313.
Article7. Cvoro A, Dundjerski J, Trajković D, Matić G. Heat stress affects the glucocorticoid receptor interaction with heat shock protein Hsp70 in the rat liver. Biochem Mol Biol Int. 1998. 46:63–70.
Article8. Drummond IAS, Steinhardt RA. The role of oxidative stress in the induction of Drosophila heat-shock proteins. Exp Cell Res. 1987. 173:439–449.
Article9. Dwyer BE, Nishimura RN, Brown IR. Synthesis of the major inducible heat shock protein in rat hippocampus after neonatal hypoxia-ischemia. Exp Neurol. 1989. 104:28–31.
Article10. Evdonin AL, Guzhova IV, Margulis BA, Medvedeva ND. Extracellular heat shock protein 70 mediates heat stress-induced epidermal growth factor receptor transactivation in A431 carcinoma cells. FEBS Lett. 2006. 580:6674–6678.
Article11. Feder ME, Hofmann GE. Heat-shock proteins, molecular chaperones, and the stress response: evolutionary and ecological physiology. Annu Rev Physiol. 1999. 61:243–282.
Article12. Fujikake N, Nagai Y, Popiel HA, Kano H, Yamaguchi M, Toda T. Alternative splicing regulates the transcriptional activity of Drosophila heat shock transcription factor in response to heat/cold stress. FEBS Lett. 2005. 579:3842–3848.
Article13. Granger DN, Höllwarth ME, Parks DA. Ischemia-reperfusion injury: role of oxygen-derived free radicals. Acta Physiol Scand Suppl. 1986. 548:47–63.14. Gullo CA, Teoh G. Heat shock proteins: to present or not, that is the question. Immunol Lett. 2004. 94:1–10.
Article15. Jabiry-Zieniewicz Z, Bobrowska K, Kaminski P, Wielgos M, Zieniewicz K, Krawczyk M. Low-dose hormonal contraception after liver transplantation. Transplant Proc. 2007. 39:1530–1532.
Article16. Kieran D, Kalmar B, Dick JRT, Riddoch-Contreras J, Burnstock G, Greensmith L. Treatment with arimoclomol, a coinducer of heat shock proteins, delays disease progression in ALS mice. Nat Med. 2004. 10:402–405.
Article17. Kwon SB, Young C, Kim DS, Choi HO, Kim KH, Chung JH, Eun HC, Park KC, Oh CK, Seo JS. Impaired repair ability of hsp70.1 KO mouse after UVB irradiation. J Dermatol Sci. 2002. 28:144–151.18. Li HM, Niki T, Taira T, Iguchi-Ariga SMM, Ariga H. Association of DJ-1 with chaperones and enhanced association and colocalization with mitochondrial Hsp70 by oxidative stress. Free Radic Res. 2005. 39:1091–1099.
Article19. Li Y, Sun P, Wang Z, Bao ED. Relationship between pathological lesion and distribution of HSP27 in transport stressed pigs. Nanjing Nong Ye Da Xue Xue Bao. 2005. 28:83–87.20. Lindquist S, Craig EA. The heat-shock proteins. Annu Rev Genet. 1988. 22:631–677.
Article21. Locke M, Tanguay RM. Increased HSF activation in muscles with a high constitutive Hsp70 expression. Cell Stress Chaperones. 1996. 1:189–196.
Article22. Locke M, Tanguay RM, Ianuzzo CD. Constitutive expression of HSP 72 in swine heart. J Mol Cell Cardiol. 1996. 28:467–474.
Article23. McGarry TJ, Lindquist S. The preferential translation of Drosophila hsp70 mRNA requires sequences in the untranslated leader. Cell. 1985. 42:903–911.
Article24. Miranda-de la Lama GC, Rivero L, Chacón G, Garcia-Belenguer S, Villarroel M, María GA. Effect of the pre-slaughter logistic chain on some indicators of welfare in lambs. Livest Sci. 2010. 128:52–59.
Article25. Morimoto RI. Cells in stress: transcriptional activation of heat shock genes. Science. 1993. 259:1409–1410.
Article26. Morimoto RI. Regulation of the heat shock transcriptional response: cross talk between a family of heat shock factors, molecular chaperones, and negative regulators. Genes Dev. 1998. 12:3788–3796.
Article27. Nakamura K, Rokutan K, Marui N, Aoike A, Kawai K. Induction of heat shock proteins and their implication in protection against ethanol-induced damage in cultured guinea pig gastric mucosal cells. Gastroenterology. 1991. 101:161–166.
Article28. Odashima M, Otaka M, Jin M, Konishi N, Sato T, Kato S, Matsuhashi T, Nakamura C, Watanabe S. Induction of a 72-kDa heat-shock protein in cultured rat gastric mucosal cells and rat gastric mucosa by zinc L-carnosine. Dig Dis Sci. 2002. 47:2799–2804.29. Otaka M, Odashima M, Tamaki K, Watanabe S. Expression and function of stress (heat shock) proteins in gastrointestinal tract. Int J Hyperthermia. 2009. 25:634–640.
Article30. Oyake J, Otaka M, Matsuhashi T, Jin M, Odashima M, Komatsu K, Wada I, Horikawa Y, Ohba R, Hatakeyama N, Itoh H, Watanabe S. Over-expression of 70-kDa heat shock protein confers protection against monochloramine-induced gastric mucosal cell injury. Life Sci. 2006. 79:300–305.
Article31. Pérez MP, Palacio J, Santolaria MP, Aceña MC, Chacón G, Gascón M, Calvo JH, Zaragoza P, Beltran JA, García-Belenguer S. Effect of transport time on welfare and meat quality in pigs. Meat Sci. 2002. 61:425–433.
Article32. Pilon N, Daneau I, Paradis V, Hamel F, Lussier JG, Viger RS, Silversides DW. Porcine SRY promoter is a target for steroidogenic factor 1. Biol Reprod. 2003. 68:1098–1106.33. Pirkkala L, Nykänen P, Sistonen L. Roles of the heat shock transcription factors in regulation of the heat shock response and beyond. FASEB J. 2001. 15:1118–1131.
Article34. Radosavljević T, Mladenović D, Vučević D, Petrović J, Hrnčić D, Djuric D, Lončar-Stevanović H, Stanojlović O. Effect of acute lindane and alcohol intoxication on serum concentration of enzymes and fatty acids in rats. Food Chem Toxicol. 2008. 46:1739–1743.
Article35. Shichijo K, Ihara M, Matsuu M, Ito M, Okumura Y, Sekine I. Overexpression of heat shock protein 70 in stomach of stress-induced gastric ulcer-resistant rats. Dig Dis Sci. 2003. 48:340–348.36. Vecerek V, Grbalova S, Voslarova E, Janackova B, Malena M. Effects of travel distance and the season of the year on death rates of broilers transported to poultry processing plants. Poult Sci. 2006. 85:1881–1884.
Article37. Whitley D, Goldberg SP, Jordan WD. Heat shock proteins: a review of the molecular chaperones. J Vasc Surg. 1999. 29:748–751.
Article38. Ye X, Meeker HC, Kozlowski PB, Wegiel J, Wang KC, Imaki H, Carp RI. Pathological changes in the liver of a senescence accelerated mouse strain (SAMP8): a mouse model for the study of liver diseases. Histol Histopathol. 2004. 19:1141–1151.39. Yu H, Bao ED, Zhao RQ, Lv QX. Effect of transportation stress on heat shock protein 70 concentration and mRNA expression in heart and kidney tissues and serum enzyme activities and hormone concentrations of pigs. Am J Vet Res. 2007. 68:1145–1150.
Article40. Yun JK, McCormick TS, Villabona C, Judware RR, Espinosa MB, Lapetina EG. Inflammatory mediators are perpetuated in macrophages resistant to apoptosis induced by hypoxia. Proc Natl Acad Sci USA. 1997. 94:13903–13908.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Differential expression of two stress-inducible hsp70 genes by various stressors
- The expression of FAS-associated factor 1 and heat shock protein 70 in ovarian cancer
- Effect of Steroid Hormones on the Expression of c-Fos, CREB, ATF, and HSP70 in Rat Uterus
- The Change in Circadian Rhythms in P301S Transgenic Mice is Linked to Variability in Hsp70-related Tau Disaggregation
- Changes of Gene Expression in NIH3T3 Cells Exposed to Osmotic and Oxidative Stresses