Obstet Gynecol Sci.
2014 Jul;57(4):281-290. 10.5468/ogs.2014.57.4.281.
The expression of FAS-associated factor 1 and heat shock protein 70 in ovarian cancer
- Affiliations
-
- 1Mizpark Obstetrics and Gynecology, Incheon, Korea.
- 2Department of Obstetrics and Gynecology, Ewha Womans University School of Medicine, Seoul, Korea. mhsmhs@ewha.ac.kr
- KMID: 1841539
- DOI: http://doi.org/10.5468/ogs.2014.57.4.281
Abstract
OBJECTIVE
In this study, we evaluated the expression of FAS-associated factor 1 (FAF1) and heat shock protein 70 (HSP70) in normal ovary and ovarian cancer, and also analyzed the correlation between FAF1 and HSP70 in ovarian cancer.
METHODS
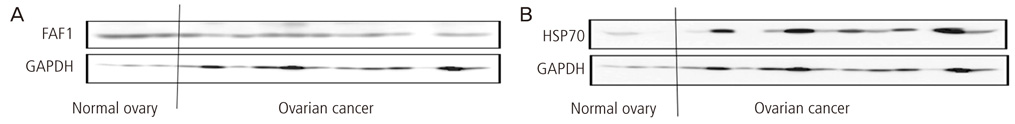
The patient group consisted of 29 unrelated Korean women diagnosed as ovarian cancers and control samples were obtained from 7 patients who underwent oophorectomy for benign disease of uterus, and normal ovary was confirmed histologically from biopsy. We examined FAF1 and HSP70 expression by western blot analysis and immunohistochemical staining in normal ovary and ovarian cancer. Furthermore, we examined a correlation between FAF1 and HSP70 in ovarian cancer.
RESULTS
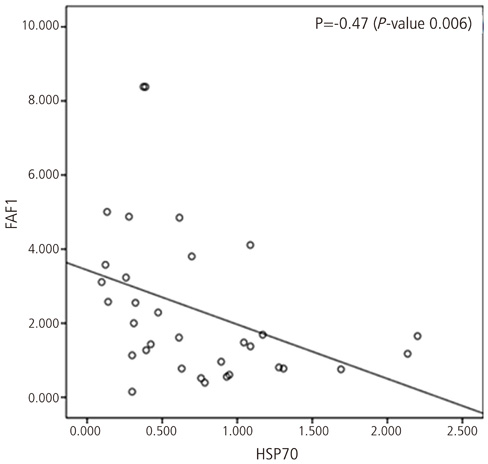
The expression of FAF1 was lower in ovarian cancer than that in normal ovary (P=0.02), and the expression of HSP70 was increased in ovarian cancer in comparison to that in normal ovary (P=0.03). The expression of FAF1 was decreased in advanced stages (stage III or stage IV) as compared with early stages (stage I or stage II) (P=0.01). The expression of HSP70 was not significantly related with ovarian cancer histology (P=0.10), but the expression of HSP70 was most increased with papillary serous carcinomas and undifferentiated ovarian cancer. The expression of FAF1 was inversely correlated with the expression of HSP70 in ovarian cancer (Spearman correlation coefficience=-0.47).
CONCLUSION
We concluded that the expression of FAF1 or HSP70 each seems to have a meaning as a biomarker for early detection of ovarian cancer. The expressions of FAF1 and HSP70 seem to be more valuable in predicting ovarian cancer when used together because of their inverse correlation. This is the first study about the expression of FAF1 in ovarian cancer and the correlation between FAF1 and HSP70 expression in ovarian cancer.
MeSH Terms
Figure
Reference
-
1. American Cancer Society. Cancer facts & figures. Atlanta: American Cancer Society;2010.2. National Cancer Control Institute. 2008 National cancer statistics. Goyang: National Cancer Center;2010.3. Ren J, Cai H, Li Y, Zhang X, Liu Z, Wang JS, et al. Tumor markers for early detection of ovarian cancer. Expert Rev Mol Diagn. 2010; 10:787–798.4. Kabawat SE, Bast RC Jr, Bhan AK, Welch WR, Knapp RC, Colvin RB. Tissue distribution of a coelomic-epithelium-related antigen recognized by the monoclonal antibody OC125. Int J Gynecol Pathol. 1983; 2:275–285.5. Jacobs I, Bast RC Jr. The CA 125 tumour-associated antigen: a review of the literature. Hum Reprod. 1989; 4:1–12.6. Berek JS, Bast RC Jr. Ovarian cancer screening. The use of serial complementary tumor markers to improve sensitivity and specificity for early detection. Cancer. 1995; 76:10 Suppl. 2092–2096.7. Gadducci A, Ferdeghini M, Prontera C, Moretti L, Mariani G, Bianchi R, et al. The concomitant determination of different tumor markers in patients with epithelial ovarian cancer and benign ovarian masses: relevance for differential diagnosis. Gynecol Oncol. 1992; 44:147–154.8. Anderson GL. Ovarian cancer biomarker screening: still too early to tell. . Womens Health (Lond Engl). 2010; 6:487–490.9. Yu JW, Shi Y. FLIP and the death effector domain family. Oncogene. 2008; 27:6216–6227.10. Chu K, Niu X, Williams LT. A Fas-associated protein factor, FAF1, potentiates Fas-mediated apoptosis. Proc Natl Acad Sci U S A. 1995; 92:11894–11898.11. Ryu SW, Kim E. Apoptosis induced by human Fas-associated factor 1, hFAF1, requires its ubiquitin homologous domain, but not the Fas-binding domain. Biochem Biophys Res Commun. 2001; 286:1027–1032.12. Menges CW, Altomare DA, Testa JR. FAS-associated factor 1 (FAF1): diverse functions and implications for oncogenesis. Cell Cycle. 2009; 8:2528–2534.13. Bjorling-Poulsen M, Seitz G, Guerra B, Issinger OG. The pro-apoptotic FAS-associated factor 1 is specifically reduced in human gastric carcinomas. Int J Oncol. 2003; 23:1015–1023.14. Hartl FU. Molecular chaperones in cellular protein folding. Nature. 1996; 381:571–579.15. Bukau B, Horwich AL. The Hsp70 and Hsp60 chaperone machines. Cell. 1998; 92:351–366.16. Chuma M, Sakamoto M, Yamazaki K, Ohta T, Ohki M, Asaka M, et al. Expression profiling in multistage hepatocarcinogenesis: identification of HSP70 as a molecular marker of early hepatocellular carcinoma. Hepatology. 2003; 37:198–207.17. Kim HJ, Song EJ, Lee YS, Kim E, Lee KJ. Human Fas-associated factor 1 interacts with heat shock protein 70 and negatively regulates chaperone activity. J Biol Chem. 2005; 280:8125–8133.18. Bea S, Salaverria I, Armengol L, Pinyol M, Fernandez V, Hartmann EM, et al. Uniparental disomies, homozygous deletions, amplifications, and target genes in mantle cell lymphoma revealed by integrative high-resolution whole-genome profiling. Blood. 2009; 113:3059–3069.19. Weersma RK, Stokkers PC, Cleynen I, Wolfkamp SC, Henckaerts L, Schreiber S, et al. Confirmation of multiple Crohn's disease susceptibility loci in a large Dutch-Belgian cohort. Am J Gastroenterol. 2009; 104:630–638.20. Hidalgo A, Baudis M, Petersen I, Arreola H, Pina P, Vazquez-Ortiz G, et al. Microarray comparative genomic hybridization detection of chromosomal imbalances in uterine cervix carcinoma. BMC Cancer. 2005; 5:77.21. Suto R, Srivastava PK. A mechanism for the specific immunogenicity of heat shock protein-chaperoned peptides. Science. 1995; 269:1585–1588.22. Asea A, Kraeft SK, Kurt-Jones EA, Stevenson MA, Chen LB, Finberg RW, et al. HSP70 stimulates cytokine production through a CD14-dependant pathway, demonstrating its dual role as a chaperone and cytokine. Nat Med. 2000; 6:435–442.23. Jaattela M. Overexpression of major heat shock protein hsp70 inhibits tumor necrosis factor-induced activation of phospholipase A2. J Immunol. 1993; 151:4286–4294.24. Soti C, Csermely P. Molecular chaperones in the etiology and therapy of cancer. Pathol Oncol Res. 1998; 4:316–321.25. Nylandsted J, Gyrd-Hansen M, Danielewicz A, Fehrenbacher N, Lademann U, Hoyer-Hansen M, et al. Heat shock protein 70 promotes cell survival by inhibiting lysosomal membrane permeabilization. J Exp Med. 2004; 200:425–435.26. Proskuryakov SY, Konoplyannikov AG, Gabai VL. Necrosis: a specific form of programmed cell death? Exp Cell Res. 2003; 283:1–16.27. Kaur J, Das SN, Srivastava A, Ralhan R. Cell surface expression of 70 kDa heat shock protein in human oral dysplasia and squamous cell carcinoma: correlation with clinicopathological features. Oral Oncol. 1998; 34:93–98.28. Trieb K, Thurnher D, Bakroeva M, Kotz R, Kornfehl J. Reversible downregulation of telomerase activity by hyperthermia in osteosarcoma cells. Int J Hyperthermia. 2000; 16:445–448.29. Park MY, Jang HD, Lee SY, Lee KJ, Kim E. Fas-associated factor-1 inhibits nuclear factor-kappaB (NF-kappaB) activity by interfering with nuclear translocation of the RelA (p65) subunit of NF-kappaB. J Biol Chem. 2004; 279:2544–2549.30. McConkey DJ, Zhu K. Mechanisms of proteasome inhibitor action and resistance in cancer. Drug Resist Updat. 2008; 11:164–179.31. Baud V, Karin M. Is NF-kappaB a good target for cancer therapy? Hopes and pitfalls. Nat Rev Drug Discov. 2009; 8:33–40.32. Fan XM, Wong BC, Wang WP, Zhou XM, Cho CH, Yuen ST, et al. Inhibition of proteasome function induced apoptosis in gastric cancer. Int J Cancer. 2001; 93:481–488.33. Birle DC, Hedley DW. Suppression of the hypoxia-inducible factor-1 response in cervical carcinoma xenografts by proteasome inhibitors. Cancer Res. 2007; 67:1735–1743.34. Yang DT, Young KH, Kahl BS, Markovina S, Miyamoto S. Prevalence of bortezomib-resistant constitutive NF-kappaB activity in mantle cell lymphoma. Mol Cancer. 2008; 7:40.35. Lazaris AC, Chatzigianni EB, Panoussopoulos D, Tzimas GN, Davaris PS, Golematis BC. Proliferating cell nuclear antigen and heat shock protein 70 immunolocalization in invasive ductal breast cancer not otherwise specified. Breast Cancer Res Treat. 1997; 43:43–51.36. Athanassiadou P, Petrakakou E, Sakelariou V, Zerva C, Liossi A, Michalas S, et al. Expression of p53, bcl-2 and heat shock protein (hsp72) in malignant and benign ovarian tumours. Eur J Cancer Prev. 1998; 7:225–231.37. Kim KK, Jang TJ, Kim JR. HSP70 and ER expression in cervical intraepithelial neoplasia and cervical cancer. J Korean Med Sci. 1998; 13:383–388.38. Nanbu K, Konishi I, Mandai M, Kuroda H, Hamid AA, Komatsu T, et al. Prognostic significance of heat shock proteins HSP70 and HSP90 in endometrial carcinomas. Cancer Detect Prev. 1998; 22:549–555.39. Bonay M, Soler P, Riquet M, Battesti JP, Hance AJ, Tazi A. Expression of heat shock proteins in human lung and lung cancers. Am J Respir Cell Mol Biol. 1994; 10:453–461.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Expression of Heat Shock Protein 70 m-RNA in Rat Bladder Overdistended by Diuresis
- Environmental factors regulating the expression of Porphyromonas gingivalis heat shock protein
- The Protective Effect of Induced Heat Shock Protein in Human Corneal Epithelial Cells
- Expression of Heat Shock Protein 70 Family in Melanocytes
- Effect of Cryopreservation on the Heat Shock Protein 90 Expression in Mouse Ovarian Tissue