Korean J Radiol.
2012 Aug;13(4):458-466. 10.3348/kjr.2012.13.4.458.
Differential Diagnosis of Axillary Inflammatory and Metastatic Lymph Nodes in Rabbit Models by Using Diffusion-Weighted Imaging: Compared with Conventional Magnetic Resonance Imaging
- Affiliations
-
- 1Department of Radiology, Tianjin Medical University General Hospital, Tianjin 300052, China. wangjunping_tj@163.com
- 2Department of Radiology, The First Affiliated Hospital of Nanchang University, Jiangxi 330006, China.
- KMID: 1383858
- DOI: http://doi.org/10.3348/kjr.2012.13.4.458
Abstract
OBJECTIVE
This experiment aims to determine the diagnostic value of diffusion-weighted imaging (DWI) in the differentiation of axillary inflammatory lymph nodes from metastatic lymph nodes in rabbit models in comparison with conventional magnetic resonance imaging (MRI).
MATERIALS AND METHODS
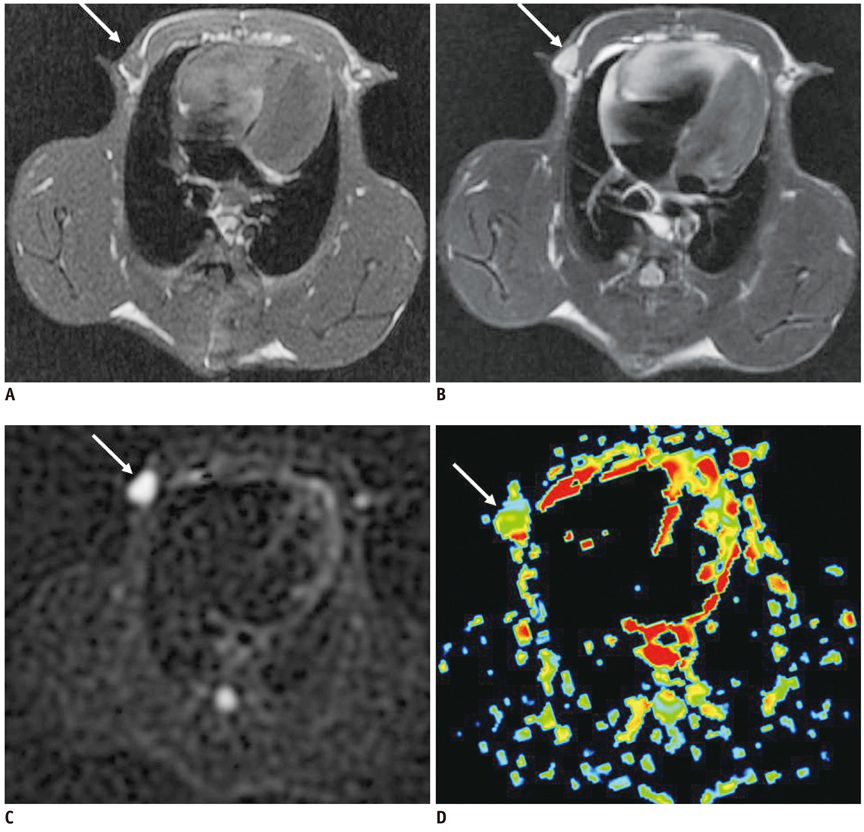
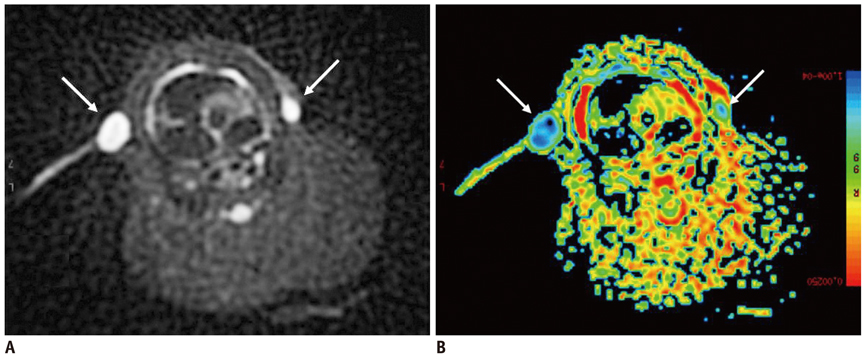
Conventional MRI and DWI were performed at 4 weeks after successful inoculation into the forty female New Zealand white rabbits' mammary glands. The size-based and signal-intensity-based criteria and the relative apparent diffusion coefficient (rADC) value were compared between the axillary inflammatory lymph nodes and metastatic lymph nodes, with histopathological findings as the reference standard. Receiver operating characteristic (ROC) curve analysis was performed to evaluate the diagnostic performance of the aforementioned criteria and rADC value in differentiating the axillary inflammatory lymph nodes from metastatic lymph nodes.
RESULTS
Thirty-two axillary inflammatory lymph nodes and 46 metastatic ones were successfully isolated and taken into pathological analysis. The differences of the aforementioned criteria between the two groups were not statistically significant (p > 0.05). However, the rADC value of the inflammatory lymph nodes (0.9 +/- 0.14) was higher than that of metastatic ones (0.7 +/- 0.18), with significant difference (p = 0.016). When the rADC value was chosen as 0.80, the area under the ROC curve is greater than all other criteria, and the sensitivity, specificity, accuracy, positive predictive value, and negative predictive value for differentiating two groups were 86.2%, 79.3%, 81.2%, 84.2%, and 85.6%, respectively.
CONCLUSION
Diffusion-weighted imaging is a promising new technique for differentiating axillary inflammatory lymph nodes from metastatic lymph nodes. Compared with routine magnetic resonance sequences, DWI could provide more useful physiological and functional information for diagnosis.
MeSH Terms
Figure
Cited by 1 articles
-
Breast Magnetic Resonance Imaging for Assessment of Internal Mammary Lymph Node Status in Breast Cancer
Hyung Won Lee, Sung Hun Kim
J Breast Cancer. 2016;19(2):191-198. doi: 10.4048/jbc.2016.19.2.191.
Reference
-
1. Orel SG, Schnall MD. MR imaging of the breast for the detection, diagnosis, and staging of breast cancer. Radiology. 2001. 220:13–30.2. Morrow M. Role of axillary dissection in breast cancer management. Ann Surg Oncol. 1996. 3:233–234.3. Stets C, Brandt S, Wallis F, Buchmann J, Gilbert FJ, Heywang-Köbrunner SH. Axillary lymph node metastases: a statistical analysis of various parameters in MRI with USPIO. J Magn Reson Imaging. 2002. 16:60–68.4. Michel SC, Keller TM, Fröhlich JM, Fink D, Caduff R, Seifert B, et al. Preoperative breast cancer staging: MR imaging of the axilla with ultrasmall superparamagnetic iron oxide enhancement. Radiology. 2002. 225:527–536.5. Pressman PI. Surgical treatment and lymphedema. Cancer. 1998. 83:2782–2787.6. Yeoh EK, Denham JW, Davies SA, Spittle MF. Primary breast cancer. Complications of axillary management. Acta Radiol Oncol. 1986. 25:105–108.7. Shin JH, Choi HY, Moon BI, Sung SH. In vitro sonographic evaluation of sentinel lymph nodes for detecting metastasis in breast cancer: comparison with histopathologic results. J Ultrasound Med. 2004. 23:923–928.8. Shetty MK, Carpenter WS. Sonographic evaluation of isolated abnormal axillary lymph nodes identified on mammograms. J Ultrasound Med. 2004. 23:63–71.9. Krishnamurthy S, Sneige N, Bedi DG, Edieken BS, Fornage BD, Kuerer HM, et al. Role of ultrasound-guided fine-needle aspiration of indeterminate and suspicious axillary lymph nodes in the initial staging of breast carcinoma. Cancer. 2002. 95:982–988.10. Steinkamp HJ, Cornehl M, Hosten N, Pegios W, Vogl T, Felix R. Cervical lymphadenopathy: ratio of long- to short-axis diameter as a predictor of malignancy. Br J Radiol. 1995. 68:266–270.11. Roth Y, Tichler T, Kostenich G, Ruiz-Cabello J, Maier SE, Cohen JS, et al. High-b-value diffusion-weighted MR imaging for pretreatment prediction and early monitoring of tumor response to therapy in mice. Radiology. 2004. 232:685–692.12. Squillaci E, Manenti G, Cova M, Di Roma M, Miano R, Palmieri G, et al. Correlation of diffusion-weighted MR imaging with cellularity of renal tumours. Anticancer Res. 2004. 24:4175–4179.13. de Bondt RB, Hoeberigs MC, Nelemans PJ, Deserno WM, Peutz-Kootstra C, Kremer B, et al. Diagnostic accuracy and additional value of diffusion-weighted imaging for discrimination of malignant cervical lymph nodes in head and neck squamous cell carcinoma. Neuroradiology. 2009. 51:183–192.14. Ono K, Ochiai R, Yoshida T, Kitagawa M, Omagari J, Kobayashi H, et al. Comparison of diffusion-weighted MRI and 2-[fluorine-18]-fluoro-2-deoxy-D-glucose positron emission tomography (FDG-PET) for detecting primary colorectal cancer and regional lymph node metastases. J Magn Reson Imaging. 2009. 29:336–340.15. Heo SH, Jeong YY, Shin SS, Kim JW, Lim HS, Lee JH, et al. Apparent diffusion coefficient value of diffusion-weighted imaging for hepatocellular carcinoma: correlation with the histologic differentiation and the expression of vascular endothelial growth factor. Korean J Radiol. 2010. 11:295–303.16. Kim JK, Kim KA, Park BW, Kim N, Cho KS. Feasibility of diffusion-weighted imaging in the differentiation of metastatic from nonmetastatic lymph nodes: early experience. J Magn Reson Imaging. 2008. 28:714–719.17. Park SO, Kim JK, Kim KA, Park BW, Kim N, Cho G, et al. Relative apparent diffusion coefficient: determination of reference site and validation of benefit for detecting metastatic lymph nodes in uterine cervical cancer. J Magn Reson Imaging. 2009. 29:383–390.18. Choi EK, Kim JK, Choi HJ, Park SH, Park BW, Kim N, et al. Node-by-node correlation between MR and PET/CT in patients with uterine cervical cancer: diffusion-weighted imaging versus size-based criteria on T2WI. Eur Radiol. 2009. 19:2024–2032.19. Lin G, Ho KC, Wang JJ, Ng KK, Wai YY, Chen YT, et al. Detection of lymph node metastasis in cervical and uterine cancers by diffusion-weighted magnetic resonance imaging at 3T. J Magn Reson Imaging. 2008. 28:128–135.20. Nakai G, Matsuki M, Inada Y, Tatsugami F, Tanikake M, Narabayashi I, et al. Detection and evaluation of pelvic lymph nodes in patients with gynecologic malignancies using body diffusion-weighted magnetic resonance imaging. J Comput Assist Tomogr. 2008. 32:764–768.21. Nakai G, Matsuki M, Harada T, Tanigawa N, Yamada T, Barentsz J, et al. Evaluation of axillary lymph nodes by diffusion-weighted MRI using ultrasmall superparamagnetic iron oxide in patients with breast cancer: Initial clinical experience. J Magn Reson Imaging. 2011. [Epub ahead of print].22. Chen JH, Ling R, Yao Q, Li Y, Chen T, Wang Z, et al. Effect of small-sized liposomal Adriamycin administered by various routes on a metastatic breast cancer model. Endocr Relat Cancer. 2005. 12:93–100.23. van den Brekel MW, Castelijns JA, Snow GB. The size of lymph nodes in the neck on sonograms as a radiologic criterion for metastasis: how reliable is it? AJNR Am J Neuroradiol. 1998. 19:695–700.24. Kim SH, Kim SC, Choi BI, Han MC. Uterine cervical carcinoma: evaluation of pelvic lymph node metastasis with MR imaging. Radiology. 1994. 190:807–811.25. Williams AD, Cousins C, Soutter WP, Mubashar M, Peters AM, Dina R, et al. Detection of pelvic lymph node metastases in gynecologic malignancy: a comparison of CT, MR imaging, and positron emission tomography. AJR Am J Roentgenol. 2001. 177:343–348.26. Obwegeser R, Lorenz K, Hohlagschwandtner M, Czerwenka K, Schneider B, Kubista E. Axillary lymph nodes in breast cancer: is size related to metastatic involvement? World J Surg. 2000. 24:546–550.27. Som PM. Detection of metastasis in cervical lymph nodes: CT and MR criteria and differential diagnosis. AJR Am J Roentgenol. 1992. 158:961–969.28. Vandecaveye V, De Keyzer F, Vander Poorten V, Dirix P, Verbeken E, Nuyts S, et al. Head and neck squamous cell carcinoma: value of diffusion-weighted MR imaging for nodal staging. Radiology. 2009. 251:134–146.29. Kim JK, Jang YJ, Cho G. Multidisciplinary functional MR imaging for prostate cancer. Korean J Radiol. 2009. 10:535–551.30. Koh DM, Collins DJ. Diffusion-weighted MRI in the body: applications and challenges in oncology. AJR Am J Roentgenol. 2007. 188:1622–1635.31. Rahbar H, Partridge SC, Eby PR, Demartini WB, Gutierrez RL, Peacock S, et al. Characterization of ductal carcinoma in situ on diffusion weighted breast MRI. Eur Radiol. 2011. 21:2011–2019.32. Imamura T, Isomoto I, Sueyoshi E, Yano H, Uga T, Abe K, et al. Diagnostic performance of ADC for Non-mass-like breast lesions on MR imaging. Magn Reson Med Sci. 2010. 9:217–225.33. Jeh SK, Kim SH, Kim HS, Kang BJ, Jeong SH, Yim HW, et al. Correlation of the apparent diffusion coefficient value and dynamic magnetic resonance imaging findings with prognostic factors in invasive ductal carcinoma. J Magn Reson Imaging. 2011. 33:102–109.34. Kul S, Cansu A, Alhan E, Dinc H, Gunes G, Reis A. Contribution of diffusion-weighted imaging to dynamic contrast-enhanced MRI in the characterization of breast tumors. AJR Am J Roentgenol. 2011. 196:210–217.35. DeLano MC, Cooper TG, Siebert JE, Potchen MJ, Kuppusamy K. High-b-value diffusion-weighted MR imaging of adult brain: image contrast and apparent diffusion coefficient map features. AJNR Am J Neuroradiol. 2000. 21:1830–1836.36. Mulkern RV, Barnes AS, Haker SJ, Hung YP, Rybicki FJ, Maier SE, et al. Biexponential characterization of prostate tissue water diffusion decay curves over an extended b-factor range. Magn Reson Imaging. 2006. 24:563–568.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- RE: Differential Diagnosis of Axillary Inflammatory and Metastatic Lymph Nodes in Rabbit Models by Using Diffusion-Weighted Imaging: Compared with Conventional Magnetic Resonance Imaging
- RE: Diffusion-Weighted Imaging of Prostate Cancer: How Can We Use It Accurately?
- Bone Involvement of Diffuse Large B Cell Lymphoma (DLBCL) Showing Unusual Manifestations Mimicking Chronic Osteomyelitis in a 58-Year-Old Man: Case Report and Clinical Application of Diffusion Weighted Magnetic Resonance Imaging
- Diffusion-Weighted Magnetic Resonance Imaging Findings in a Patient with Trigeminal Ganglioneuroma
- The Usefulness of Diffusion-weighted MR Imaging for Differentiation between Degenerative Spines and Infectious Spondylitis