J Korean Med Sci.
2012 May;27(5):465-470. 10.3346/jkms.2012.27.5.465.
Depletion of Mitochondrial DNA Stabilizes C1qTNF-Related Protein 6 mRNA in Muscle Cells
- Affiliations
-
- 1Department of Biochemistry, Dongguk University School of Medicine, Gyeongju, Korea. psyoon@dongguk.ac.kr
- KMID: 1372785
- DOI: http://doi.org/10.3346/jkms.2012.27.5.465
Abstract
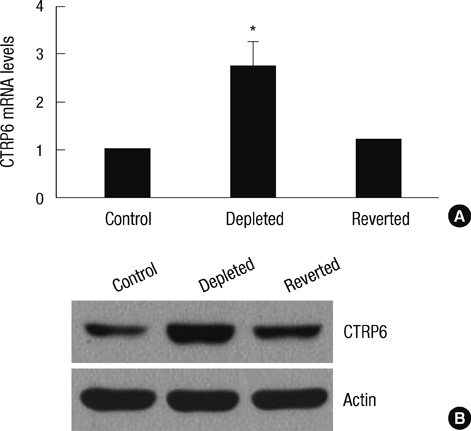
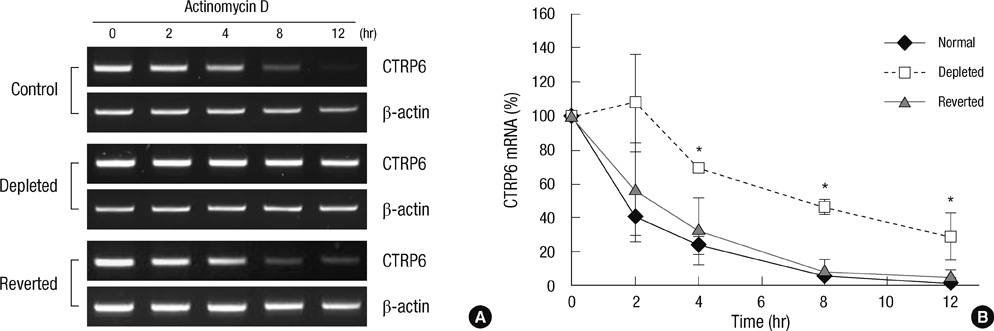
- Mutation and reduction of mitochondrial DNA (mtDNA) have been suggested as factors in the pathogenesis of several metabolic diseases. Recently, we demonstrated that C1qTNF-related protein-6 (CTRP6) is involved in fatty acid metabolism in muscle cells. In this study, we showed that expression of CTRP6 was up-regulated in mtDNA-depleted C2C12 cells, which displayed a marked decrease in cellular mtDNA and ATP content. Replacement of mtDNA normalized the expression level of CTRP6 similar to that in normal C2C12 cells, indicating that CTRP6 expression was up-regulated by mtDNA depletion. However, CTRP6 promoter activity remained unchanged in mtDNA-depleted cells. We also found that mtDNA depletion inhibited decay of CTRP6 mRNA. Taken together, mtDNA depletion induces an increase in CTRP6 expression by increasing mRNA stability.
Keyword
MeSH Terms
Figure
Reference
-
1. Wong GW, Krawczyk SA, Kitidis-Mitrokostas C, Revett T, Gimeno R, Lodish HF. Molecular, biochemical and functional characterizations of C1q/TNF family members: adipose tissue-selective expression patterns, regulation by PPAR-gamma agonist, Cys-mediated oligomerizations, combinatorial associations and metabolic functions. Biochem J. 2008. 416:161–177.2. Lee W, Kim MJ, Park EJ, Choi YJ, Park SY. C1qTNF-related protein-6 mediates fatty acid oxidation via the activation of the AMP-activated protein kinase. FEBS Lett. 2010. 584:968–972.3. Kim MJ, Lee W, Park EJ, Park SY. C1qTNF-related protein-6 increases the expression of interleukin-10 in macrophages. Mol Cells. 2010. 30:59–64.4. Wallace DC. A mitochondrial paradigm of metabolic and degenerative diseases, aging, and cancer: a dawn for evolutionary medicine. Annu Rev Genet. 2005. 39:359–407.5. Park SY, Choi GH, Choi HI, Ryu J, Jung CY, Lee W. Depletion of mitochondrial DNA causes impaired glucose utilization and insulin resistance in L6 GLUT4myc myocytes. J Biol Chem. 2005. 280:9855–9864.6. Epstein CB, Waddle JA, Hale W 4th, Davé V, Thornton J, Macatee TL, Garner HR, Butow RA. Genome-wide responses to mitochondrial dysfunction. Mol Biol Cell. 2001. 12:297–308.7. Biswas G, Anandatheerthavarada HK, Zaidi M, Avadhani NG. Mitochondria to nucleus stress signaling: a distinctive mechanism of NFkappaB/Rel activation through calcineurin-mediated inactivation of IkappaB-beta. J Cell Biol. 2003. 161:507–519.8. Biswas G, Guha M, Avadhani NG. Mitochondria-to-nucleus stress signaling in mammalian cells: nature of nuclear gene targets, transcription regulation, and induced resistance to apoptosis. Gene. 2005. 354:132–139.9. Garcia-Ruiz I, Rodriguez-Juan C, Diaz-Sanjuan T, del Hoyo P, Colina F, Muñoz-Yagüe T, Solís-Herruzo JA. Uric acid and anti-TNF antibody improve mitochondrial dysfunction in ob/ob mice. Hepatology. 2006. 44:581–591.10. Park SY, Choi JH, Ryu HS, Pak YK, Park KS, Lee HK, Lee W. C1q tumor necrosis factor alpha-related protein isoform 5 is increased in mitochondrial DNA-depleted myocytes and activates AMP-activated protein kinase. J Biol Chem. 2009. 284:27780–27789.11. King MP, Attardi G. Human cells lacking mtDNA: repopulation with exogenous mitochondria by complementation. Science. 1989. 246:500–503.12. Biswas G, Adebanjo OA, Freedman BD, Anandatheerthavarada HK, Vijayasarathy C, Zaidi M, Kotlikoff M, Avadhani NG. Retrograde Ca2+ signaling in C2C12 skeletal myocytes in response to mitochondrial genetic and metabolic stress: a novel mode of inter-organelle crosstalk. EMBO J. 1999. 18:522–533.13. Park SY, Ryu J, Lee W. O-GlcNAc modification on IRS-1 and Akt2 by PUGNAc inhibits their phosphorylation and induces insulin resistance in rat primary adipocytes. Exp Mol Med. 2005. 37:220–229.14. Desjardins P, Frost E, Morais R. Ethidium bromide-induced loss of mitochondrial DNA from primary chicken embryo fibroblasts. Mol Cell Biol. 1985. 5:1163–1169.15. Zylber E, Vesco C, Penman S. Selective inhibition of the synthesis of mitochondria-associated RNA by ethidium bromide. J Mol Biol. 1969. 44:195–204.16. Hayakawa T, Noda M, Yasuda K, Yorifuji H, Taniguchi S, Miwa I, Sakura H, Terauchi Y, Hayashi J, Sharp GW, et al. Ethidium bromide-induced inhibition of mitochondrial gene transcription suppresses glucose-stimulated insulin release in the mouse pancreatic beta-cell line betaHC9. J Biol Chem. 1998. 273:20300–20307.17. Amuthan G, Biswas G, Ananadatheerthavarada HK, Vijayasarathy C, Shephard HM, Avadhani NG. Mitochondrial stress-induced calcium signaling, phenotypic changes and invasive behavior in human lung carcinoma A549 cells. Oncogene. 2002. 21:7839–7849.18. Amuthan G, Biswas G, Zhang SY, Klein-Szanto A, Vijayasarathy C, Avadhani NG. Mitochondria-to-nucleus stress signaling induces phenotypic changes, tumor progression and cell invasion. EMBO J. 2001. 20:1910–1920.19. Park SY, Lee S, Park KS, Lee HK, Lee W. Proteomic analysis of cellular change involved in mitochondria-to-nucleus communication in L6 GLUT4myc myocytes. Proteomics. 2006. 6:1210–1222.20. Choi HS, Carman GM. Respiratory deficiency mediates the regulation of CHO1-encoded phosphatidylserine synthase by mRNA stability in Saccharomyces cerevisiae. J Biol Chem. 2007. 282:31217–31227.21. Lee W, Choi HI, Kim MJ, Park SY. Depletion of mitochondrial DNA upregulates the expression of MDR1 gene via an increase in mRNA stability. Exp Mol Med. 2008. 40:109–117.22. Ryu HS, Park SY, Ma D, Zhang J, Lee W. The induction of microRNA targeting IRS-1 is involved in the development of insulin resistance under conditions of mitochondrial dysfunction in hepatocytes. PLoS One. 2011. 6:e17343.23. Carling D. The AMP-activated protein kinase cascade: a unifying system for energy control. Trends Biochem Sci. 2004. 29:18–24.24. Shulman GI. Cellular mechanisms of insulin resistance. J Clin Invest. 2000. 106:171–176.25. Tomas E, Tsao TS, Saha AK, Murrey HE, Zhang Cc C, Itani SI, Lodish HF, Ruderman NB. Enhanced muscle fat oxidation and glucose transport by ACRP30 globular domain: acetyl-CoA carboxylase inhibition and AMP-activated protein kinase activation. Proc Natl Acad Sci U S A. 2002. 99:16309–16313.26. Yamauchi T, Kamon J, Minokoshi Y, Ito Y, Waki H, Uchida S, Yamashita S, Noda M, Kita S, Ueki K, et al. Adiponectin stimulates glucose utilization and fatty-acid oxidation by activating AMP-activated protein kinase. Nat Med. 2002. 8:1288–1295.27. Prigione A, Cortopassi G. Mitochondrial DNA deletions induce the adenosine monophosphate-activated protein kinase energy stress pathway and result in decreased secretion of some proteins. Aging Cell. 2007. 6:619–630.28. Koh EH, Park JY, Park HS, Jeon MJ, Ryu JW, Kim M, Kim SY, Kim MS, Kim SW, Park IS, et al. Essential role of mitochondrial function in adiponectin synthesis in adipocytes. Diabetes. 2007. 56:2973–2981.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Depletion of mitochondrial DNA up-regulates the expression of MDR1 gene via an increase in mRNA stability
- MPV17-related Hepatocerebral Mitochondrial DNA Depletion Syndrome
- Mitochondrial myopathies caused by prolonged use of telbivudine
- Alteration of mitochondrial DNA content modulates antioxidant enzyme expressions and oxidative stress in myoblasts
- Effects of isorhamnetin on the regulation of mitochondrial function in C2C12 muscle cells