Ann Clin Neurophysiol.
2017 Jan;19(1):40-45. 10.14253/acn.2017.19.1.40.
Mitochondrial myopathies caused by prolonged use of telbivudine
- Affiliations
-
- 1Department of Neurology, Research Institute for Convergence of Biomedical Science and Technology, Pusan National University Yangsan Hospital, Pusan National University School of Medicine, Yangsan, Korea. dskim@pusan.ac.kr
- 2Department of Neurology, Pusan National University School of Medicine, Yangsan, Korea.
- KMID: 2367993
- DOI: http://doi.org/10.14253/acn.2017.19.1.40
Abstract
- BACKGROUND
Telbivudine is a nucleoside analogue used for the treatment of chronic hepatitis B, but it often develops mitochondrial toxicity leading to symptomatic myopathy. In this study, three patients with telbivudine induced myopathy were enrolled in order to investigate the nature and pathogenesis of mitochondrial toxicity caused by long-term use of telbivudine.
METHODS
Clinical features, laboratory findings, muscle pathology, and quantitation of mitochondrial DNA were studied in three patients.
RESULTS
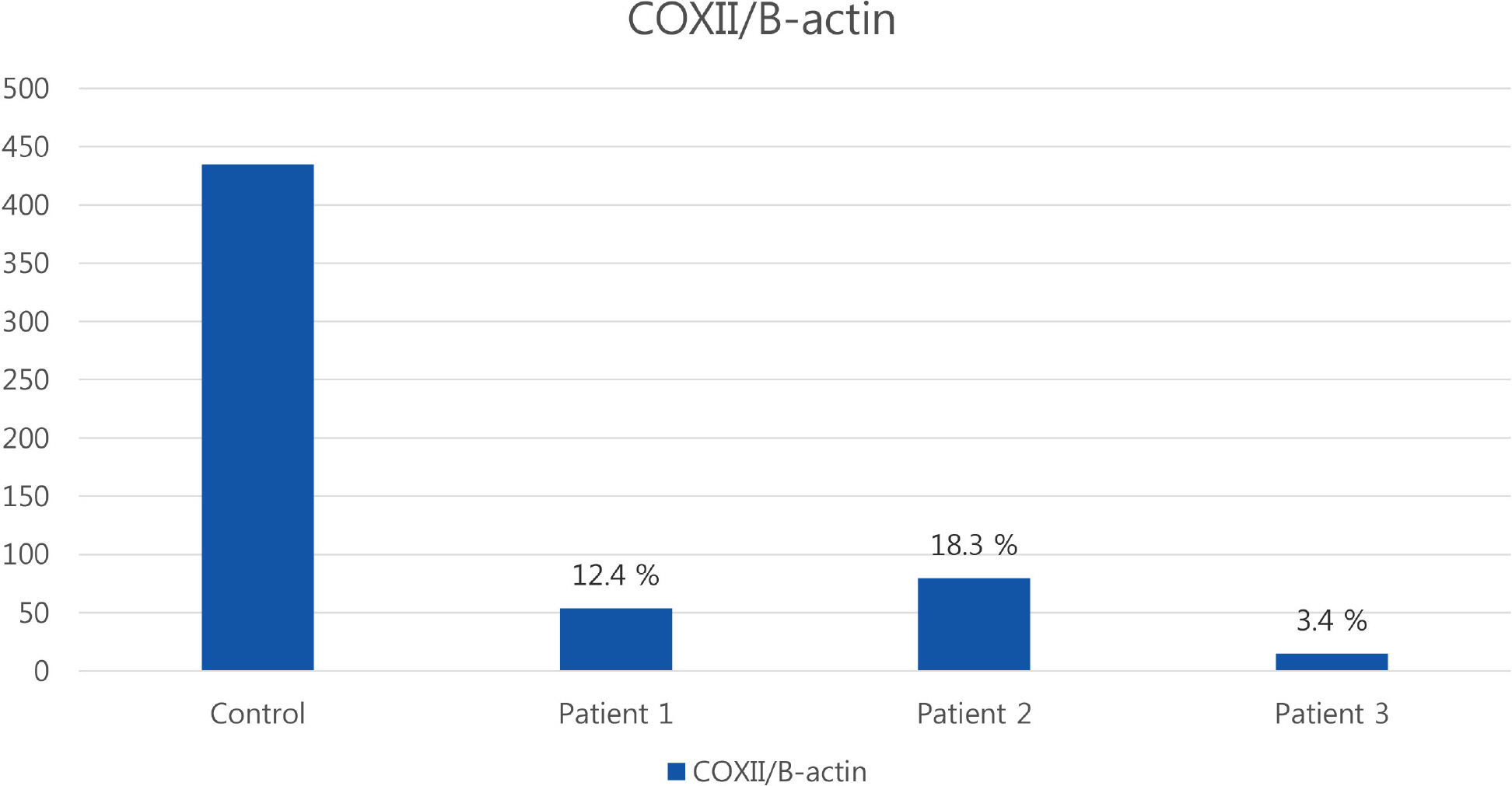
Patients presented with progressive muscle weakness with high serum creatine kinase levels. Light microscopic findings of muscle pathology showed ragged red fibers that reacted strongly with succinate dehydrogenase stain, but negative for cytochrome c oxidase activities. Electron microscopy revealed abnormal mitochondrial accumulation with rod shaped inclusions. The quantitative peroxidase chain reaction showed a depletion of mitochondrial DNA in skeletal muscle of the patients.
CONCLUSIONS
Nucleoside analogues including telbivudine are potent inhibitors of viral DNA polymerases. However, they are not specific for viral DNA and can disturb mitochondrial replication at the same time. All nucleotide analogues should be used with close clinical observation in order to avoid development of mitochondrial myopathy.
Keyword
MeSH Terms
-
Creatine Kinase
DNA, Mitochondrial
DNA, Viral
Electron Transport Complex IV
Hepatitis B, Chronic
Humans
Microscopy, Electron
Mitochondrial Myopathies*
Muscle Weakness
Muscle, Skeletal
Muscular Diseases
Pathology
Peroxidase
Succinate Dehydrogenase
Creatine Kinase
DNA, Mitochondrial
DNA, Viral
Electron Transport Complex IV
Peroxidase
Succinate Dehydrogenase
Figure
Reference
-
1.McMahon BJ. The natural history of chronic hepatitis B virus infection. Hepatology. 2009. 49(5 Suppl):S45–S55.
Article2.Fattovich G., Bortolotti F., Donato F. Natural history of chronic hepatitis B: special emphasis on disease progression and prognostic factors. J Hepatol. 2008. 48:335–352.
Article3.Liaw YF., Gane E., Leung N., Zeuzem S., Wang Y., Lai CL, et al. 2-year GLOBE trial results: telbivudine is superior to lamivudine in patients with chronic hepatitis B. Gastroenterology. 2009. 136:486–495.
Article4.Zou XJ., Jiang XQ., Tian DY. Clinical features and risk factors of creatine kinase elevations and myopathy associated with telbivudine. J Viral Hepat. 2011. 18:892–896.
Article5.Seok JI., Lee DK., Lee CH., Park MS., Kim SY., Kim HS, et al. Long-term therapy with clevudine for chronic hepatitis B can be associated with myopathy characterized by depletion of mitochondrial DNA. Hepatology. 2009. 49:2080–2086.
Article6.Masanés F., Barrientos A., Cebrián M., Pedrol E., Miró O., Casade-mont J, et al. Clinical, histological and molecular reversibility of zidovudine myopathy. J Neurol Sci. 1998. 159:226–228.
Article7.Kim EH., Park H., Lee KH., Ahn SH., Kim SM., Han KH. Two cases of telbivudine-induced myopathy in siblings with chronic hepatitis B. Clin Mol Hepatol. 2013. 19:82–86.
Article8.Wang M., Da Y., Cai H., Lu Y., Wu L., Jia J. Telbivudine myopathy in a patient with chronic hepatitis B. Int J Clin Pharm. 2012. 34:422–425.
Article9.Dalakas MC., Illa I., Pezeshkpour GH., Laukaitis JP., Cohen B., Griffin JL. Mitochondrial myopathy caused by long-term zidovudine therapy. N Engl J Med. 1990. 322:1098–1105.
Article10.Lo YL., See SJ., Tan CK. Continuous single motor unit electromyo-graphic activity in adefovir associated myopathy. J Clin Neurosci. 2008. 15:1073–1074.
Article11.Ambang T., Tan JS., Ong S., Wong KT., Goh KJ. Clinicopatholog-ical features of telbivudine-associated myopathy. PLoS ONE. 2016. 11:e0162760.
Article12.Mazzucco CE., Hamatake RK., Colonno RJ., Tenney DJ. Entecavir for treatment of hepatitis B virus displays no in vitro mitochondrial toxicity or DNA polymerase gamma inhibition. Antimicrob Agents Chemother. 2008. 52:598–605.
Article13.Yuan K., Guochun W., Huang Z., Lin B., Zhou H., Lu X. Entecavir-as-sociated myopathy: a case report and literature review. Muscle Nerve. 2014. 49:610–614.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Genetics of Mitochondrial Myopathies
- Anesthetic Management of a Patient with Mitochondrial Myopathy Undergoing Orthopedic Surgery
- A Case of Mitochondrial Myopathy With Cardiac Involvement
- A Case of Early Onset MELAS Patient with Wolff-Parkinson-White Syndrome
- Vascular Hyperemia and Crossed Cerebellar Diaschisis in MELAS Patient Presented as Stroke-Like Episode and Seizure