J Vet Sci.
2010 Jun;11(2):93-101. 10.4142/jvs.2010.11.2.93.
Microtubule distribution in somatic cell nuclear transfer bovine embryos following control of nuclear remodeling type
- Affiliations
-
- 1College of Veterinary Medicine and Institute of Veterinary Science, Kangwon National University, Chuncheon 200-701, Korea. htcheong@kangwon.ac.kr
- 2College of Animal Life Sciences, Kangwon National University, Chuncheon 200-701, Korea.
- KMID: 1110854
- DOI: http://doi.org/10.4142/jvs.2010.11.2.93
Abstract
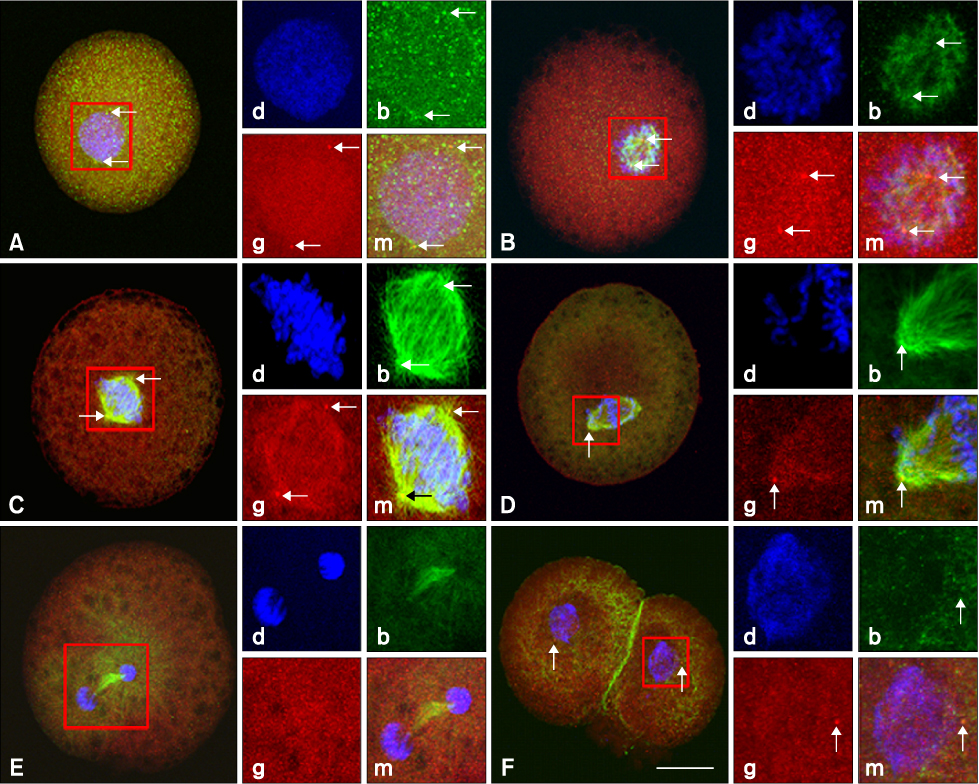
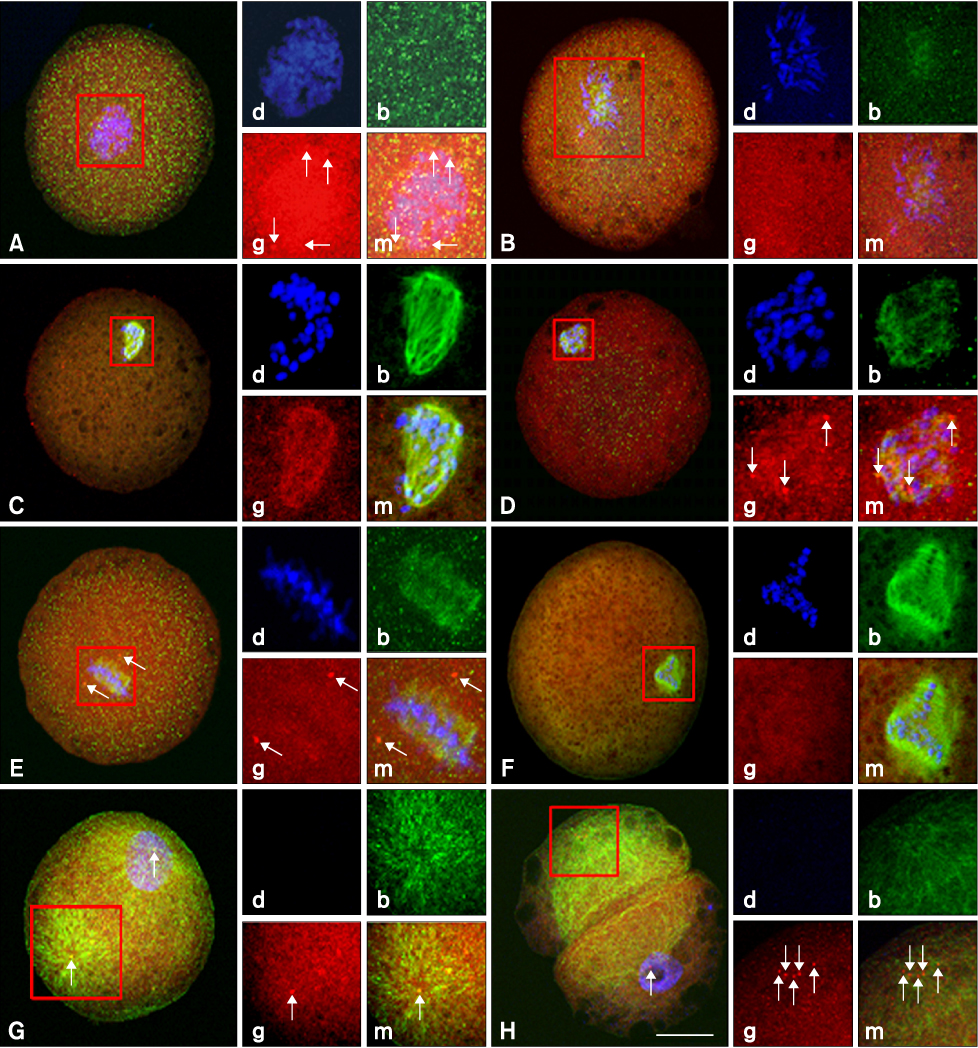
- This study was conducted to evaluate the microtubule distribution following control of nuclear remodeling by treatment of bovine somatic cell nuclear transfer (SCNT) embryos with caffeine or roscovitine. Bovine somatic cells were fused to enucleated oocytes treated with either 5 mM caffeine or 150 micrometer roscovitine to control the type of nuclear remodeling. The proportion of embryos that underwent premature chromosome condensation (PCC) was increased by caffeine treatment but was reduced by roscovitine treatment (p < 0.05). The microtubule organization was examined by immunostaining beta- and gamma-tubulins at 15 min, 3 h, and 20 h of fusion using laser scanning confocal microscopy. The gamma-tubulin foci inherited from the donor centrosome were observed in most of the SCNT embryos at 15 min of fusion (91.3%) and most of them did not disappear until 3 h after fusion, regardless of treatment (82.9-87.2%). A significantly high proportion of embryos showing an abnormal chromosome or microtubule distribution was observed in the roscovitine-treated group (40.0%, p < 0.05) compared to the caffeine-treated group (22.1%). In conclusion, PCC is a favorable condition for the normal organization of microtubules, and inhibition of PCC can cause abnormal mitotic division of bovine SCNT embryos by causing microtubule dysfunction.
Keyword
MeSH Terms
-
Animals
Caffeine/pharmacology
Cattle/embryology/*physiology
Cell Nucleus/drug effects/*physiology/ultrastructure
Female
Fertilization in Vitro/veterinary
Male
Microscopy, Confocal/veterinary
Microtubules/drug effects/*physiology/ultrastructure
Nuclear Transfer Techniques/veterinary
Oocytes/*physiology
Pregnancy
Purines/pharmacology
Figure
Cited by 1 articles
-
Mitochondrial and DNA damage in bovine somatic cell nuclear transfer embryos
In-Sun Hwang, Hyo-Kyung Bae, Hee-Tae Cheong
J Vet Sci. 2013;14(3):235-240. doi: 10.4142/jvs.2013.14.3.235.
Reference
-
1. Brackett BG, Oliphant G. Capacitation of rabbit spermatozoa in vitro. Biol Reprod. 1975. 12:260–274.2. Cheong HT, Takahashi Y, Kanagawa H. Birth of mice after transplantation of early cell-cycle-stage embryonic nuclei into enucleated oocytes. Biol Reprod. 1993. 48:958–963.
Article3. Cheong HT, Takahashi Y, Kanagawa H. Relationship between nuclear remodeling and subsequent development of mouse embryonic nuclei transferred to enucleated oocytes. Mol Reprod Dev. 1994. 37:138–145.
Article4. Choi JY, Kim CI, Park CK, Yang BK, Cheong HT. Effect of activation time on the nuclear remodeling and in vitro development of nuclear transfer embryos derived from bovine somatic cells. Mol Reprod Dev. 2004. 69:289–295.
Article5. Collas P, Robl JM. Relationship between nuclear remodeling and development in nuclear transplant rabbit embryos. Biol Reprod. 1991. 45:455–465.
Article6. Dai Y, Wang L, Wang H, Liu Y, Li N, Lyu Q, Keefe DL, Albertini DF, Liu L. Fate of centrosomes following somatic cell nuclear transfer (SCNT) in bovine oocytes. Reproduction. 2006. 131:1051–1061.
Article7. Ito J, Hirabayashi M, Kato M, Takeuchi A, Ito M, Shimada M, Hochi S. Contribution of high p34cdc2 kinase activity to premature chromosome condensation of injected somatic cell nuclei in rat oocytes. Reproduction. 2005. 129:171–180.
Article8. Ju JC, Tsay C, Ruan CW. Alterations and reversibility in the chromatin, cytoskeleton and development of pig oocytes treated with roscovitine. Mol Reprod Dev. 2003. 64:482–491.
Article9. Kalt A, Schliwa M. Molecular components of the centrosome. Trends Cell Biol. 1993. 3:118–128.
Article10. Kellogg DR, Moritz M, Alberts BM. The centrosome and cellular organization. Annu Rev Biochem. 1994. 63:639–674.
Article11. Kikuchi K, Naito K, Noguchi J, Shimada A, Kaneko H, Yamashita M, Aoki F, Tojo H, Toyoda Y. Maturation/M-phase promoting factor: a regulator of aging in porcine oocytes. Biol Reprod. 2000. 63:715–722.
Article12. Kim JM, Ogura A, Nagata M, Aoki F. Analysis of the mechanism for chromatin remodeling in embryos reconstructed by somatic nuclear transfer. Biol Reprod. 2002. 67:760–766.
Article13. Kwon DJ, Park CK, Yang BK, Cheong HT. Control of nuclear remodelling and subsequent in vitro development and methylation status of porcine nuclear transfer embryos. Reproduction. 2008. 135:649–656.
Article14. Lequarre AS, Marchandise J, Moreau B, Massip A, Donnay I. Cell cycle duration at the time of maternal zygotic transition for in vitro produced bovine embryos: effect of oxygen tension and transcription inhibition. Biol Reprod. 2003. 69:1707–1713.
Article15. Lonergan P, Faerge I, Hyttel PM, Boland M, Fair T. Ultrastructural modifications in bovine oocytes maintained in meiotic arrest in vitro using roscovitine or butyrolactone. Mol Reprod Dev. 2003. 64:369–378.
Article16. Ma W, Zhang D, Hou Y, Li YH, Sun QY, Sun XF, Wang WH. Reduced expression of MAD2, BCL2, and MAP kinase activity in pig oocytes after in vitro aging are associated with defects in sister chromatid segregation during meiosis II and embryo fragmentation after activation. Biol Reprod. 2005. 72:373–383.
Article17. Miki H, Inoue K, Ogonuki N, Mochida K, Nagashima H, Baba T, Ogura A. Cytoplasmic asters are required for progression past the first cell cycle in cloned mouse embryos. Biol Reprod. 2004. 71:2022–2028.
Article18. Moudjou M, Bordes N, Paintrand M, Bornens M. γ-Tubulin in mammalian cells: The centrosomal and the cytosolic forms. J Cell Sci. 1996. 109:875–887.19. Park JH, Choi YL, Kwon DJ, Hwang IS, Park CK, Yang BK, Cheong HT. Control of MPF activity of recipient oocytes and subsequent development and DNA methylation of somatic cell nuclear transfer bovine embryos. Reprod Dev Biol. 2009. 33:223–228.20. Raff JW, Kellogg DR, Alberts BM. Drosophila γ-tubulin is part of a complex containing two previously identified centrosomal MAPs. J Cell Biol. 1993. 121:823–835.
Article21. Rosenkrans CF Jr, First NL. Culture of bovine zygotes to the blastocyst stage: effects of amino acids and vitamins. Theriogenology. 1991. 35:266.
Article22. Salisbury JL. Centrin, centrosomes, and mitotic spindle poles. Curr Opin Cell Biol. 1995. 7:39–45.
Article23. Shin MR, Park SW, Shim H, Kim NH. Nuclear and microtubule reorganization in nuclear-transferred bovine embryos. Mol Reprod Dev. 2002. 62:74–82.
Article24. Stearns T, Evans L, Kirschner M. γ-Tubulin is a highly conserved component of the centrosome. Cell. 1991. 65:825–836.
Article25. Tani T, Kato Y, Tsunoda Y. Direct exposure of chromosomes to nonactivated ovum cytoplasm is effective for bovine somatic cell nucleus reprogramming. Biol Reprod. 2001. 64:324–330.
Article26. Tani T, Kato Y, Tsunoda Y. Reprogramming of bovine somatic cell nuclei is not directly regulated by maturation promoting factor or mitogen-activated protein kinase activity. Biol Reprod. 2003. 69:1890–1894.
Article27. Wakayama T, Perry ACF, Zuccotti M, Johnson KR, Yanagimachi R. Full-term development of mice from enucleated oocytes injected with cumulus cell nuclei. Nature. 1998. 394:69–74.
Article28. Wilmut I, Beaujean N, de Sousa PA, Dinnyes A, King TJ, Paterson LA, Wells DN, Young LE. Somatic cell nuclear transfer. Nature. 2002. 419:583–586.
Article29. Yin XJ, Tani T, Yonemura I, Kawakami M, Miyamoto K, Hasegawa R, Kato Y, Tsunoda Y. Production of cloned pigs from adult somatic cells by chemically assisted removal of maternal chromosomes. Biol Reprod. 2002. 67:442–446.
Article30. Zheng Y, Wong ML, Alberts B, Mitchison T. Nucleation of microtubule assembly by a γ-tubulin-containing ring complex. Nature. 1995. 378:578–583.
Article31. Zhong Z, Spate L, Hao Y, Li R, Lai L, Katayama M, Sun QY, Prather RS, Schatten H. Remodeling of centrosomes in intraspecies and interspecies nuclear transfer porcine embryos. Cell Cycle. 2007. 6:1510–1521.
Article32. Zhong ZS, Zhang G, Meng XQ, Zhang YL, Chen DY, Schatten H, Sun QY. Function of donor cell centrosome in intraspecies and interspecies nuclear transfer embryos. Exp Cell Res. 2005. 306:35–46.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Mitochondrial and DNA damage in bovine somatic cell nuclear transfer embryos
- Production of cloned sei whale (Balaenoptera borealis) embryos by interspecies somatic cell nuclear transfer using enucleated pig oocytes
- Expression of polo-like kinase 1 in pre-implantation stage murine somatic cell nuclear transfer embryos
- Identification of abnormal gene expression in bovine transgenic somatic cell nuclear transfer embryos
- Cloned calves derived from somatic cell nuclear transfer embryos cultured in chemically defined medium or modified synthetic oviduct fluid