J Vet Sci.
2010 Mar;11(1):43-50. 10.4142/jvs.2010.11.1.43.
Ethanol extract of Angelica gigas inhibits croton oil-induced inflammation by suppressing the cyclooxygenase - prostaglandin pathway
- Affiliations
-
- 1College of Veterinary Medicine and Research Institute of Veterinary Medicine, Chungbuk National University, Cheongju 361-763, Korea. solar93@cbu.ac.kr
- 2College of Pharmacy, Chungbuk National University, Cheongju 361-763, Korea. solar93@cbu.ac.kr
- 3Daejeon Health Sciences College, Daejeon 300-711, Korea.
- KMID: 1106156
- DOI: http://doi.org/10.4142/jvs.2010.11.1.43
Abstract
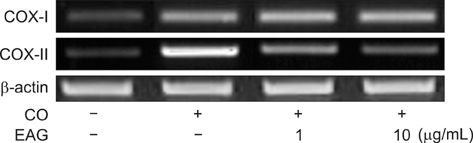
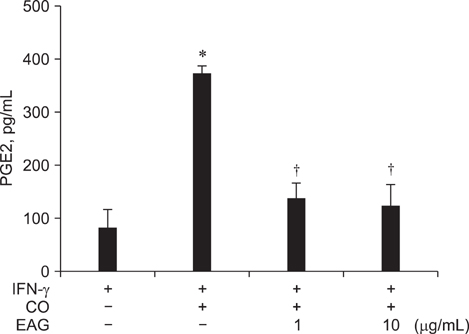
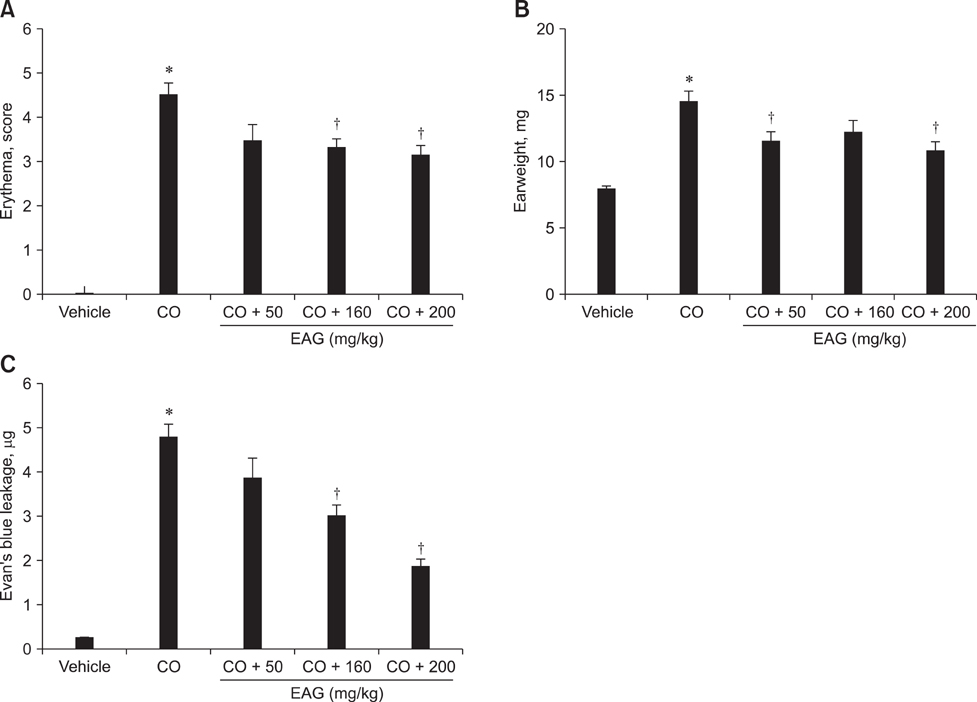
- The anti-inflammatory effects of an ethanol extract of Angelica gigas (EAG) were investigated in vitro and in vivo using croton oil-induced inflammation models. Croton oil (20 microgram/mL) up-regulated mRNA expression of cyclooxygenase (COX)-I and COX-II in the macrophage cell line, RAW 264.7, resulting in the release of high concentrations of prostaglandin E2 (PGE2). EAG (1~10 microgram/mL) markedly suppressed croton oil-induced COX-II mRNA expression and PGE2 production. Application of croton oil (5% in acetone) to mouse ears caused severe local erythema, edema and vascular leakage, which were significantly attenuated by oral pre-treatment with EAG (50~500 mg/kg). Croton oil dramatically increased blood levels of interleukin (IL)-6 and PGE2 without affecting tumor-necrosis factor (TNF)-alpha and nitric oxide (NO) levels. EAG pre-treatment remarkably lowered IL-6 and PGE2, but did not alter TNF-alpha or NO concentrations. These results indicate that EAG attenuates inflammatory responses in part by blocking the COX-PGE2 pathway. Therefore, EAG could be a promising candidate for the treatment of inflammatory diseases.
MeSH Terms
-
Angelica/*immunology
Animals
Cell Line
Cyclooxygenase 1/genetics/*immunology
Cyclooxygenase 2/genetics/*immunology
Dinoprostone/genetics/immunology
Inflammation/drug therapy/enzymology/*immunology
Interleukin-6/blood
Macrophages
Male
Mice
Mice, Inbred ICR
Nitric Oxide/blood
Phytotherapy/*methods
Plant Extracts/*pharmacology/therapeutic use
Plant Roots/immunology
RNA, Messenger/chemistry/genetics
Reverse Transcriptase Polymerase Chain Reaction
Tumor Necrosis Factor-alpha/blood
Figure
Reference
-
1. Adams V, Nehrhoff B, Späte U, Linke A, Schulze PC, Baur A, Gielen S, Hambrecht R, Schuler G. Induction of iNOS expression in skeletal muscle by IL-1β and NFκB activation: an in vitro and in vivo study. Cardiovasc Res. 2002. 54:95–104.
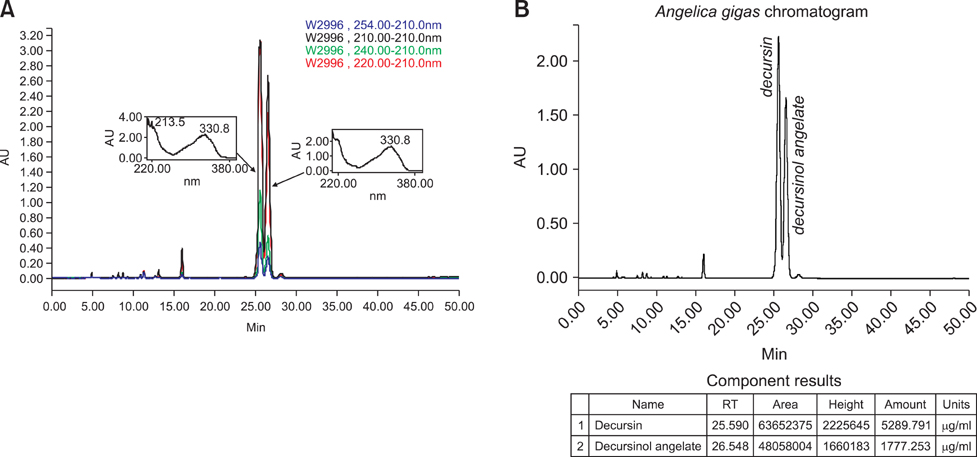
Article2. Ahn KS, Sim WS, Lee IK, Seu YB, Kim IH. Decursinol angelate: a cytotoxic and protein kinase C activating agent from the root of Angelica gigas. Planta Med. 1997. 63:360–361.
Article3. Bae EA, Han MJ, Kim NJ, Kim DH. Anti-Helicobacter pylori activity of herbal medicines. Biol Pharm Bull. 1998. 21:990–992.
Article4. Bresnihan B. Rheumatoid arthritis: principles of early treatment. J Rheumatol Suppl. 2002. 66:9–12.5. Chabaud M, Fossiez F, Taupin JL, Miossec P. Enhancing effect of IL-17 on IL-1-induced IL-6 and leukemia inhibitory factor production by rheumatoid arthritis synoviocytes and its regulation by Th2 cytokines. J Immunol. 1998. 161:409–414.6. Chi HJ, Kim HS. Studies on the components of Umbelliferae plants in Korea: Pharmacological study of decursin, decursinol and nodakenin. Korean J Pharmacol. 1970. 1:25–32.7. Cho S, Takahashi M, Toita S, Cyong JC. Suppression of adjuvant arthritis on rat by oriental herbs. Shoyakugaku Zasshi. 1982. 36:78–81.8. Choi EK, Park D, Yon JM, Hur GH, Ha YC, Che JH, Kim J, Shin S, Jang JY, Hwang SY, Seong YH, Kim DJ, Kim JC, Kim YB. Protection by sustained release of physostigmine and procyclidine of soman poisoning in rats. Eur J Pharmacol. 2004. 505:83–91.
Article9. Choi SS, Han KJ, Lee JK, Lee HK, Han EJ, Kim DH, Suh HW. Antinociceptive mechanisms of orally administered decursinol in the mouse. Life Sci. 2003. 73:471–485.
Article10. Day R. Adverse reactions to TNF-α inhibitors in rheumatoid arthritis. Lancet. 2002. 359:540–541.
Article11. Eigler A, Sinha B, Hartmann G, Endres S. Taming TNF: strategies to restrain this proinflammatory cytokine. Immunol Today. 1997. 18:487–492.
Article12. Ershler WB, Keller ET. Age-associated increased interleukin-6 gene expression, late-life diseases, and frailty. Annu Rev Med. 2000. 51:245–270.
Article13. Feldmann M, Brennan FM, Maini RN. Role of cytokines in rheumatoid arthritis. Annu Rev Immunol. 1996. 14:397–400.
Article14. Guslandi M. Nitric oxide and inflammatory bowel diseases. Eur J Clin Invest. 1998. 28:904–907.
Article15. Heinrich PC, Castell JV, Andus T. Interleukin-6 and the acute phase response. Biochem J. 1990. 265:621–636.
Article16. Hevel JM, Marletta MA. Nitric-oxide synthase assays. Methods Enzymol. 1994. 233:250–258.17. Hunskaar S, Hole K. The formalin test in mice: dissociation between inflammatory and non-inflammatory pain. Pain. 1987. 30:103–114.
Article18. Jiang C, Lee HJ, Li GX, Guo J, Malewicz B, Zhao Y, Lee EO, Lee HJ, Lee JH, Kim MS, Kim SH, Lu J. Potent antiandrogen and androgen receptor activities of an Angelica gigas-containing herbal formulation: identification of decursin as a novel and active compound with implications for prevention and treatment of prostate cancer. Cancer Res. 2006. 66:453–463.
Article19. Jiang J, Wu F, Lu J, Lu Z, Xu Q. Anti-inflammatory activity of the aqueous extract from Rhizoma smilacis glabrae. Pharmacol Res. 1997. 36:309–314.
Article20. Joo SS, Yoo YM, Ahn BW, Nam SY, Kim YB, Hwang KW, Lee DI. Prevention of inflammation-mediated neurotoxicity by Rg3 and its role in microglial activation. Biol Pharm Bull. 2008. 31:1392–1396.
Article21. Jung DJ, Porzel A, Huneck S. Gigasol and other coumarins isolated from Angelica gigas. Phytochemistry. 1991. 30:710–712.22. Kang SY, Lee KY, Park MJ, Kim YC, Markelonis GJ, Oh TH, Kim YC. Decursin from Angelica gigas mitigates amnesia induced by scopolamine in mice. Neurobiol Learn Mem. 2003. 79:11–18.
Article23. Kang SY, Lee KY, Sung SH, Kim YC. Four new neuroprotective dihydropyranocoumarins from Angelica gigas. J Nat Prod. 2005. 68:56–59.24. Kim HH, Bang SS, Choi JS, Han H, Kim IH. Involvement of PKC and ROS in the cytotoxic mechanism of anti-leukemic decursin and its derivatives and their structure-activity relationship in human K562 erythroleukemia and U937 myeloleukemia cells. Cancer Lett. 2005. 223:191–201.
Article25. Kim JH, Jeong JH, Jeon ST, Kim H, Ock J, Suk K, Kim SI, Song KS, Lee WH. Decursin inhibits induction of inflammatory mediators by blocking nuclear factor-κB activation in macrophages. Mol Pharmacol. 2006. 69:1783–1790.
Article26. Lee JK, Choi SS, Lee HK, Han KJ, Han EJ, Suh HW. Effects of ginsenoside Rd and decursinol on the neurotoxic responses induced by kainic acid in mice. Planta Med. 2003. 69:230–234.
Article27. Lee YY, Lee S, Jin JL, Yun-Choi HS. Platelet anti-aggregatory effects of coumarins from the roots of Angelica genuflexa and A. gigas. Arch Pharm Res. 2003. 26:723–726.
Article28. Lester RS, Knowles SR, Shear NH. The risks of systemic corticosteroid use. Dermatol Clin. 1998. 16:277–288.
Article29. Lichtenstein DR, Syngal S, Wolfe MM. Nonsteroidal antiinflammatory drugs and the gastrointestinal tract. The double-edged sword. Arthritis Rheum. 1995. 38:5–18.
Article30. Marletta MA, Yoon PS, Iyengar R, Leaf CD, Wishnok JS. Macrophage oxidation of L-arginine to nitrite and nitrate: nitric oxide is an intermediate. Biochemistry. 1998. 27:8706–8711.
Article31. Moncada S, Palmer RM, Higgs EA. Nitric oxide: physiology, pathophysiology, and pharmacology. Pharmacol Rev. 1991. 43:109–142.32. Mukherjee D, Nissen SE, Topol EJ. Risk of cardiovascular events associated with selective COX-2 inhibitors. JAMA. 2001. 286:954–959.
Article33. Ohshiro T, Namatame I, Lee EW, Kawagishi H, Tomoda H. Molecular target of decursins in the inhibition of lipid droplet accumulation in macrophages. Biol Pharm Bull. 2006. 29:981–984.
Article34. Oliveira de Melo J, da Conceição Torrado Truiti M, Muscará MN, Bolonheis SM, Dantas JA, Caparroz-Assef SM, Cuman RK, Bersani-Amado CA. Anti-inflammatory activity of crude extract and fractions of Nectandra falcifolia leaves. Biol Pharm Bull. 2006. 29:2241–2245.
Article35. Pang L, Hoult JR. Cytotoxicity to macrophages of tetrandrine, an antisilicosis alkaloid, accompanied by an overproduction of prostaglandins. Biochem Pharmacol. 1997. 53:773–782.
Article36. Park HH, Lee S, Son HY, Park SB, Kim MS, Choi EJ, Singh TS, Ha JH, Lee MG, Kim JE, Hyun MC, Kwon TK, Kim YH, Kim SH. Flavonoids inhibit histamine release and expression of proinflammatory cytokines in mast cells. Arch Pharm Res. 2008. 31:1303–1311.
Article37. Park WH, Park SY, Kim HM, Kim CH. Effect of a Korean traditional formulation, Hwaotang, on superoxide generation in human neutrophils, platelet aggregation in human blood, and nitric oxide, prostaglandin E2 production and paw oedema induced by carrageenan in mice. Immunopharmacol Immunotoxicol. 2004. 26:53–73.
Article38. Ritchlin CT, Haas-Smith SA, Li P, Hicks DG, Schwarz EM. Mechanisms of TNF-α- and RANKL-mediated osteoclastogenesis and bone resorption in psoriatic arthritis. J Clin Invest. 2003. 111:821–831.
Article39. Romano M, Faggioni R, Sironi M, Sacco S, Echtenacher B, Di Santo E, Salmona M, Ghezzi P. Carrageenan-induced acute inflammation in the mouse air pouch synovial model. Role of tumour necrosis factor. Mediators Inflamm. 1997. 6:32–38.
Article40. Seo YJ, Kwon MS, Park SH, Sim YB, Choi SM, Huh GH, Lee JK, Suh HW. The analgesic effect of decursinol. Arch Pharm Res. 2009. 32:937–943.
Article41. Shin S, Jeon JH, Park D, Jang JY, Joo SS, Hwang BY, Choe SY, Kim YB. Anti-inflammatory effects of an ethanol extract of Angelica gigas in a Carrageenan-air pouch inflammation model. Exp Anim. 2009. 58:431–436.
Article42. Shin S, Park D, Jeon JH, Kim JS, Park SK, Kim YB. Anti-inflammatory effects of an ethanolic extract of Angelica gigas in a thermal burn model. J Biomed Res. 2008. 9:29–36.43. Tanaka S, Kano Y, Tabata M, Konoshima M. Effects of "Toki" (Angelica acutiloba Kitagawa) extracts on writhing and capillary permeability in mice (analgesic and antiinflammatory effects). Yakugaku Zasshi. 1971. 91:1098–1104.
Article44. Van Snick J. Interleukin-6: an overview. Annu Rev Immunol. 1990. 8:253–278.
Article45. Wallace JL, Chapman K, McKnight W. Limited anti-inflammatory efficacy of cyclo-oxygenase-2 inhibition in carrageenan-airpouch inflammation. Br J Pharmacol. 1999. 126:1200–1204.
Article46. Xie QW, Whisnant R, Nathan C. Promoter of the mouse gene encoding calcium-independent nitric oxide synthase confers inducibility by interferon γ and bacterial lipopolysaccharide. J Exp Med. 1993. 177:1779–1784.
Article47. Yan JJ, Kim DH, Moon YS, Jung JS, Ahn EM, Baek NI, Song DK. Protection against β-amyloid peptide-induced memory impairment with long-term administration of extract of Angelica gigas or decursinol in mice. Prog Neuropsychopharmacol Biol Psychiatry. 2004. 28:25–30.
Article48. Yui Y, Hattori R, Kosuga K, Eizawa H, Hiki K, Kawai C. Purification of nitric oxide synthase from rat macrophage. J Biol Chem. 1991. 266:12544–12547.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- The Ethanol Extract of Croton Seed Inhibits the Oral Pathogen, Streptococcus mutans
- Effects of Armeniacae Semen and Amygdalin on the Lipopolysaccaride-induced Prostaglandin E2 Synthesis and Nitric Oxide Production in Mouse BV2 Microglial Cells
- An ethanolic extract of Angelica gigas improves atherosclerosis by inhibiting vascular smooth muscle cell proliferation
- Targeting Cyclooxygenase 2 and HER - 2 / neu Pathways Inhibits Colorectal Carcinoma Growth
- A combinational effect of acetaminophen and oriental herbs on the regulation of inflammatory mediators in microglia cell line, BV2