Yonsei Med J.
2009 Oct;50(5):689-696. 10.3349/ymj.2009.50.5.689.
Survival Rate and Neurological Outcome after Operation for Advanced Spinal Metastasis (Tomita's Classification > or = Type 4)
- Affiliations
-
- 1Department of Neurosurgery, Spine and Spinal Cord Institute, Gangnam Severance Spine Hospital, Yonsei University College of Medicine, Seoul, Korea. spinekks@yuhs.ac
- KMID: 1103824
- DOI: http://doi.org/10.3349/ymj.2009.50.5.689
Abstract
- PURPOSE
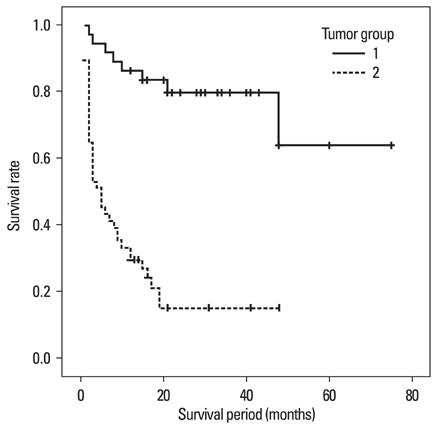
We investigated whether primary malignancy entities and the extent of tumor resection have an effect on the survival rate and neurological improvement in patients with spinal metastases that extend beyond the vertebral compartment (Tomita's classification > or = type 4). MATERIALS AND METHODS: We retrospectively reviewed 87 patients with advanced spinal metastasis who underwent surgery. They were divided into groups 1 and 2 according to whether they responded to adjuvant therapy or not, respectively. They were subdivided according to the extent of tumor resection: group 1, gross total resection (G1GT); group 1, subtotal resection (G1ST); group 2, gross total resection (G2GT); and group 2, subtotal resection (G2ST). The origin of the tumor, survival rate, extent of resection, and neurological improvement were analyzed. RESULTS: Group 1 had a better survival rate than group 2. The G1GT subgroup showed a better prognosis than the G1ST subgroup. In group 2, the extent of tumor resection (G2GT vs. G2ST) did not affect survival rate. In all subgroups, neurological status improved one month after surgery, however, the G2ST subgroup had worsened at the last follow-up. There was no local recurrence at the last follow-up in the G1GT subgroup. Four out of 13 patients in the G2GT subgroup showed a local recurrence of spinal tumors and progressive worsening of neurological status. CONCLUSION: In patients with spinal metastases (Tomita's classification > or = type 4), individuals who underwent gross total resection of tumors that responded to adjuvant therapy showed a higher survival rate than those who underwent subtotal resection. For tumors not responding to adjuvant therapy, we suggest palliative surgical decompression.
Keyword
MeSH Terms
Figure
Cited by 1 articles
-
Patterns of Treatment for Metastatic Pathological Fractures of the Spine: The Efficacy of Each Treatment Modality
Jae Hwan Cho, Jung-Ki Ha, Chang Ju Hwang, Dong-Ho Lee, Choon Sung Lee
Clin Orthop Surg. 2015;7(4):476-482. doi: 10.4055/cios.2015.7.4.476.
Reference
-
1. Berrettoni BA, Carter JR. Mechanisms of cancer metastasis to bone. J Bone Joint Surg Am. 1986. 68:308–312.
Article2. Wise JJ, Fischgrund JS, Herkowitz HN, Montgomery D, Kurz LT. Complication, survival rates, and risk factors of surgery for metastatic disease of the spine. Spine (Phila Pa 1976). 1999. 24:1943–1951.
Article3. Bhalla SK. Metastatic disease of the spine. Clin Orthop Relat Res. 1970. 73:52–60.
Article4. Bartels RH, van der Linden YM, van der Graaf WT. Spinal extradural metastasis: review of current treatment options. CA Cancer J Clin. 2008. 58:245–259.
Article5. Perrin RG. Metastatic tumors of the axial spine. Curr Opin Oncol. 1992. 4:525–532.
Article6. Metastatic Tumors of the Spine: Diagnosis and Treatment. J Am Acad Orthop Surg. 1993. 1:76–86.7. Sheehan JP, Jagannathan J. Review of spinal radiosurgery: a minimally invasive approach for the treatment of spinal and paraspinal metastases. Neurosurg Focus. 2008. 25:E18.
Article8. Sundaresan N, Rothman A, Manhart K, Kelliher K. Surgery for solitary metastases of the spine: rationale and results of treatment. Spine (Phila Pa 1976). 2002. 27:1802–1806.9. Loblaw DA, Laperriere NJ. Emergency treatment of malignant extradural spinal cord compression: an evidence-based guideline. J Clin Oncol. 1998. 16:1613–1624.
Article10. Maranzano E, Latini P. Effectiveness of radiation therapy without surgery in metastatic spinal cord compression: final results from a prospective trial. Int J Radiat Oncol Biol Phys. 1995. 32:959–967.
Article11. Tomita K, Kawahara N, Murakami H, Demura S. Total en bloc spondylectomy for spinal tumors: improvement of the technique and its associated basic background. J Orthop Sci. 2006. 11:3–12.
Article12. Tokuhashi Y, Matsuzaki H, Toriyama S, Kawano H, Ohsaka S. Scoring system for the preoperative evaluation of metastatic spine tumor prognosis. Spine (Phila Pa 1976). 1990. 15:1110–1113.
Article13. Tomita K, Kawahara N, Kobayashi T, Yoshida A, Murakami H, Akamaru T. Surgical strategy for spinal metastases. Spine (Phila Pa 1976). 2001. 26:298–306.
Article14. Fujita T, Ueda Y, Kawahara N, Baba H, Tomita K. Local spread of metastatic vertebral tumors. A histologic study. Spine (Phila Pa 1976). 1997. 22:1905–1912.15. Bartels RH, Feuth T, van der Maazen R, Verbeek AL, Kappelle AC, André Grotenhuis J, et al. Development of a model with which to predict the life expectancy of patients with spinal epidural metastasis. Cancer. 2007. 110:2042–2049.
Article16. Cereceda LE, Flechon A, Droz JP. Management of vertebral metastases in prostate cancer: a retrospective analysis in 119 patients. Clin Prostate Cancer. 2003. 2:34–40.
Article17. Pittas AG, Adler M, Fazzari M, Tickoo S, Rosai J, Larson SM, et al. Bone metastases from thyroid carcinoma: clinical characteristics and prognostic variables in one hundred forty-six patients. Thyroid. 2000. 10:261–268.
Article18. Rades D, Veninga T, Stalpers LJ, Basic H, Rudat V, Karstens JH, et al. Outcome after radiotherapy alone for metastatic spinal cord compression in patients with oligometastases. J Clin Oncol. 2007. 25:50–56.
Article19. Frankel HL, Hancock DO, Hyslop G, Melzak J, Michaelis LS, Ungar GH, et al. The value of postural reduction in the initial management of closed injuries of the spine with paraplegia and tetraplegia. I. Paraplegia. 1969. 7:179–192.
Article20. Nather A, Bose K. The results of decompression of cord or cauda equina compression from metastatic extradural tumors. Clin Orthop Relat Res. 1982. (169):103–108.
Article21. Tokuhashi Y, Matsuzaki H, Oda H, Oshima M, Ryu J. A revised scoring system for preoperative evaluation of metastatic spine tumor prognosis. Spine (Phila Pa 1976). 2005. 30:2186–2191.
Article22. Hosono N, Ueda T, Tamura D, Aoki Y, Yoshikawa H. Prognostic relevance of clinical symptoms in patients with spinal metastases. Clin Orthop Relat Res. 2005. (436):196–201.
Article23. Tatsui H, Onomura T, Morishita S, Oketa M, Inoue T. Survival rates of patients with metastatic spinal cancer after scintigraphic detection of abnormal radioactive accumulation. Spine (Phila Pa 1976). 1996. 21:2143–2148.
Article24. Böhm P, Huber J. The surgical treatment of bony metastases of the spine and limbs. J Bone Joint Surg Br. 2002. 84:521–529.
Article25. Coleman RE. Clinical features of metastatic bone disease and risk of skeletal morbidity. Clin Cancer Res. 2006. 12:6243s–6249s.
Article26. Hirabayashi H, Ebara S, Kinoshita T, Yuzawa Y, Nakamura I, Takahashi J, et al. Clinical outcome and survival after palliative surgery for spinal metastases: palliative surgery in spinal metastases. Cancer. 2003. 97:476–484.
Article27. Patchell RA, Tibbs PA, Regine WF, Payne R, Saris S, Kryscio RJ, et al. Direct decompressive surgical resection in the treatment of spinal cord compression caused by metastatic cancer: a randomised trial. Lancet. 2005. 366:643–648.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Minimally Invasive Spinal Stabilization Using Fluoroscopic-Guided Percutaneous Screws as a Form of Palliative Surgery in Patients with Spinal Metastasis
- Analysis of Prognostic Factors Relating to Postoperative Survival in Spinal Metastases
- Postoperative Survival and Ambulatory Outcome in Metastatic Spinal Tumors : Prognostic Factor Analysis
- Survival-Related Factors of Spinal Metastasis with Hepatocellular Carcinoma in Current Surgical Treatment Modalities : A Single Institute Experience
- Clinical features and survival outcome of locally advanced extrahepatic cholangiocarcinoma