Korean J Radiol.
2010 Oct;11(5):507-513. 10.3348/kjr.2010.11.5.507.
Functional Neuroanatomy Associated with Natural and Urban Scenic Views in the Human Brain: 3.0T Functional MR Imaging
- Affiliations
-
- 1Interdisciplinary Program of Biomedical Engineering, Chonnam National University, Gwangju 500-757, Korea. gwjeong@jnu.ac.kr
- 2Department of Radiology, Chonnam National University Medical School, Gwangju 501-757, Korea.
- 3Department of Physical and Rehabilitation Medicine, Chonnam National University Medical School, Gwangju 501-757, Korea.
- 4Department of Wood Science and Engineering, Chonnam National University, Gwangju 500-757, Korea.
- 5Department of Architectural Engineering, Chonnam National University, Gwangju 500-757, Korea.
- KMID: 1102574
- DOI: http://doi.org/10.3348/kjr.2010.11.5.507
Abstract
OBJECTIVE
By using a functional magnetic resonance imaging (fMRI) technique we assessed brain activation patterns while subjects were viewing the living environments representing natural and urban scenery.
MATERIALS AND METHODS
A total of 28 healthy right-handed subjects underwent an fMRI on a 3.0 Tesla MRI scanner. The stimulation paradigm consisted of three times the rest condition and two times the activation condition, each of which lasted for 30 and 120 seconds, respectively. During the activation period, each subject viewed natural and urban scenery, respectively.
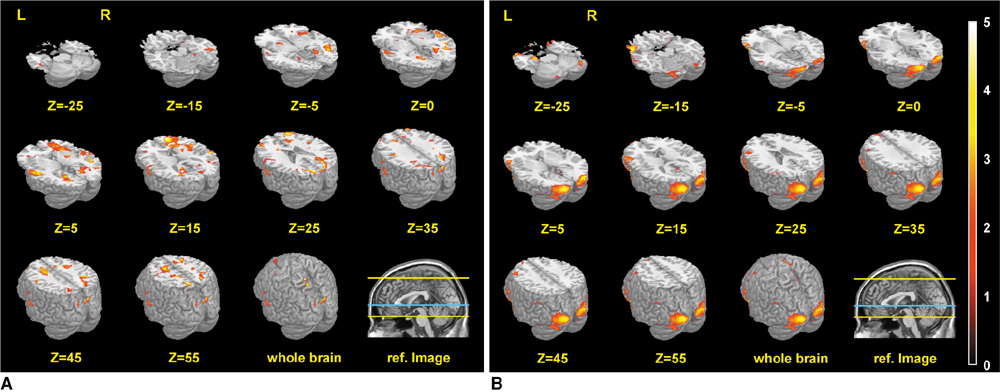
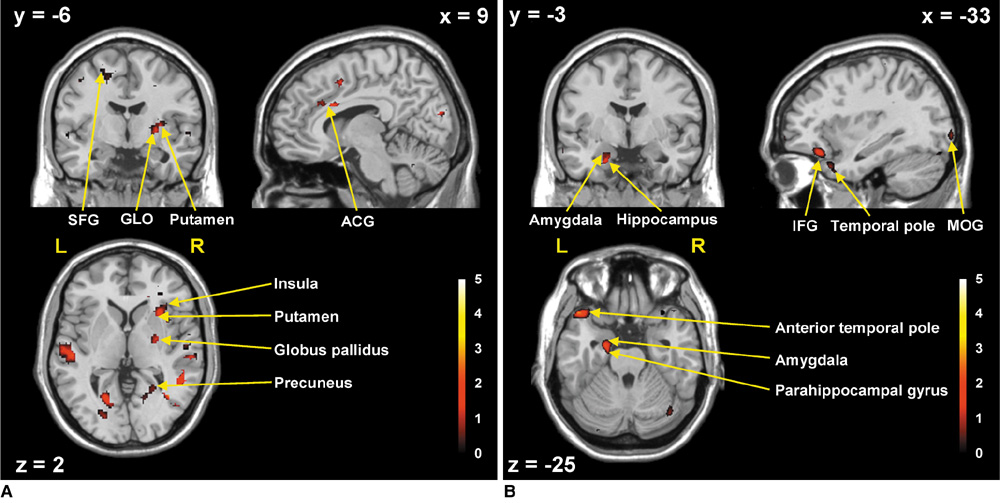
RESULTS
The predominant brain activation areas observed following exposure to natural scenic views in contrast with urban views included the superior and middle frontal gyri, superior parietal gyrus, precuneus, basal ganglia, superior occipital gyrus, anterior cingulate gyrus, superior temporal gyrus, and insula. On the other hand, the predominant brain activation areas following exposure to urban scenic views in contrast with natural scenes included the middle and inferior occipital gyri, parahippocampal gyrus, hippocampus, amygdala, anterior temporal pole, and inferior frontal gyrus.
CONCLUSION
Our findings support the idea that the differential functional neuroanatomies for each scenic view are presumably related with subjects' emotional responses to the natural and urban environment, and thus the differential functional neuroanatomy can be utilized as a neural index for the evaluation of friendliness in ecological housing.
Keyword
MeSH Terms
Figure
Cited by 1 articles
-
MRI Study on the Functional and Spatial Consistency of Resting State-Related Independent Components of the Brain Network
Bumseok Jeong, Jeewook Choi, Ji-Woong Kim
Korean J Radiol. 2012;13(3):265-274. doi: 10.3348/kjr.2012.13.3.265.
Reference
-
1. Bolund P, Hunhammar S. Ecosystem services in urban areas. Ecol Econ. 1999. 29:293–301.2. Chiesura A. The role of urban parks for the sustainable city. Landsc Urban Plan. 2004. 68:129–138.3. Kaplan R, Kaplan S. The experience of nature, a psychological perspective. 1989. Cambridge: Cambridge University Press.4. Banaka WH, Young DW. Community coping skills enhanced by an adventure camp for adult chronic psychiatric patients. Hosp Community Psychiatry. 1985. 36:746–748.5. Ulrich RS, Simons RF, Losito BD, Fiorito E, Miles MA, Zelson M. Stress recovery during exposure to natural and urban environments. J Environ Psychol. 1991. 11:201–230.6. Laumann K, Gärling T, Stormark KM. Selective attention and heart rate responses to natural and urban environments. J Environ Psychol. 2003. 23:125–134.7. Kuo FE, Sullivan WC. Environment and crime in the inner city: does vegetation reduce crime? Environ Behav. 2001. 33:343–367.8. Taylor AF, Kuo FE, Sullivan WC. Views of nature and self-discipline: evidence from inner city children. J Environ Psychol. 2002. 22:49–63.9. Ulrich RS. Natural versus urban scenes: some psychophysiological effects. Environ Behav. 1981. 13:523–556.10. Nakamura R, Fujii E. A comparative study of the characteristics of the electroencephalogram when observing a hedge and a concrete block fence. J Jpn Inst Landscape Arch. 1992. 55:139–144.11. Ogawa S, Lee TM, Nayak AS, Glynn P. Oxygenation-sensitive contrast in magnetic resonance image of rodent brain at high magnetic fields. Magn Reson Med. 1990. 14:68–78.12. Lane RD, Reiman EM, Bradley MM, Lang PJ, Ahern GL, Davidson RJ, et al. Neuroanatomical correlates of pleasant and unpleasant emotion. Neuropsychologia. 1997. 35:1437–1444.13. Lane RD, Chua PM, Dolan RJ. Common effects of emotional valence, arousal and attention on neural activation during visual processing of pictures. Neuropsychologia. 1999. 37:989–997.14. Royet JP, Zald D, Versace R, Costes N, Lavenne F, Koenig O, et al. Emotional responses to pleasant and unpleasant olfactory, visual, and auditory stimuli: a positron emission tomography study. J Neurosci. 2000. 20:7752–7759.15. Gray JR, Braver TS, Raichle ME. Integration of emotion and cognition in the lateral prefrontal cortex. Proc Natl Acad Sci U S A. 2002. 99:4115–4120.16. Kaplan S, Kaplan R, Wendt JS. Rated preference and complexity for natural and urban visual material. Percept Psychophys. 1972. 12:354–356.17. Paradiso S, Johnson DL, Andreasen NC, O'Leary DS, Watkins GL, Ponto LL, et al. Cerebral blood flow changes associated with attribution of emotional valence to pleasant, unpleasant, and neutral visual stimuli in a PET study of normal subjects. Am J Psychiatry. 1999. 156:1618–1629.18. Lane RD, Reiman EM, Ahern GL, Schwartz GE, Davidson RJ. Neuroanatomical correlates of happiness, sadness, and disgust. Am J Psychiatry. 1997. 154:926–933.19. Lang PJ, Bradley MM, Fitzsimmons JR, Cuthbert BN, Scott JD, Moulder B, et al. Emotional arousal and activation of the visual cortex: an fMRI analysis. Psychophysiology. 1998. 35:199–210.20. Zald DH, Lee JT, Fluegel KW, Pardo JV. Aversive gustatory stimulation activates limbic circuits in humans. Brain. 1998. 121:1143–1154.21. Sinha R, Lacadie C, Skudlarski P, Wexler BE. Neural circuits underlying emotional distress in humans. Ann N Y Acad Sci. 2004. 1032:254–257.22. Davachi L, Mitchell JP, Wagner AD. Multiple routes to memory: distinct medial temporal lobe processes build item and source memories. Proc Natl Acad Sci U S A. 2003. 100:2157–2162.23. Epstein R, Kanwisher N. A cortical representation of the local visual environment. Nature. 1998. 392:598–601.24. Zald DH, Pardo JV. Emotion, olfaction, and the human amygdala: amygdala activation during aversive olfactory stimulation. Proc Natl Acad Sci U S A. 1997. 94:4119–4124.25. Siebert M, Markowitsch HJ, Bartel P. Amygdala, affect and cognition: evidence from 10 patients with Urbach-Wiethe disease. Brain. 2003. 126:2627–2637.26. Nakamura K, Kubota K. The primate temporal pole: its putative role in object recognition and memory. Behav Brain Res. 1996. 77:53–77.27. Lane RD, Fink GR, Chua PM, Dolan RJ. Neural activation during selective attention to subjective emotional response. Neuroreport. 1997. 8:3969–3972.28. Dougherty DD, Shin LM, Alpert NM, Pitman RK, Orr SP, Lasko M, et al. Anger in healthy men: a PET study using script-driven imagery. Biol Psychiatry. 1999. 46:466–472.29. Kimbrell TA, George MS, Parekh PI, Ketter TA, Podell DM, Danielson AL, et al. Regional brain activity during transient self-induced anxiety and anger in healthy adults. Biol Psychiatry. 1999. 46:454–465.30. Kaplan JT, Freedman J, Iacoboni M. US versus them: political attitudes and party affiliation influence neural response to faces of presidential candidates. Neuropsychologia. 2007. 45:55–64.31. Phan KL, Wager T, Taylor SF, Liberzon I. Functional neuroanatomy of emotion: a meta-analysis of emotion activation studies in PET and fMRI. Neuroimage. 2002. 16:331–348.32. Hofer A, Siedentopf CM, Ischebeck A, Rettenbacher MA, Verius M, Felber S, et al. Gender differences in regional cerebral activity during the perception of emotion: a functional MRI study. Neuroimage. 2006. 32:854–862.33. Fletcher PC, Frith CD, Baker SC, Shallice T, Frackowiak RS, Dolan RJ. The mind's eye--precuneus activation in memory related imagery. Neuroimage. 1995. 2:195–200.34. Pessoa L, Kastner S, Ungerleider LG. Attentional control of the processing of neural and emotional stimuli. Brain Res Cogn Brain Res. 2002. 15:31–45.35. Makino Y, Yokosawa K, Takeda Y, Kumada T. Visual search and memory search engage extensive overlapping cerebral cortices: an fMRI study. Neuroimage. 2004. 23:525–533.36. Jung YC, An SK, Seok JH, Kim JS, Oh SJ, Moon DH, et al. Neural substrates associated with evaluative processing during co-activation of positivity and negativity: a PET investigation. Biol Psychol. 2006. 73:253–261.37. Reiman EM, Lane RD, Ahern GL, Schwartz GE, Davidson RJ, Friston KJ, et al. Neuroanatomical correlates of externally and internally generated human emotion. Am J Psychiatry. 1997. 154:918–925.38. Damasio AR, Grabowski TJ, Bechara A, Damasio H, Ponto LL, Parvizi J, et al. Subcortical and cortical brain activity during the feeling of self-generated emotions. Nat Neurosci. 2000. 3:1049–1056.39. Phan KL, Wager TD, Taylor SF, Liberzon I. Functional neuroimaging studies of human emotions. CNS Spectr. 2004. 9:258–266.40. Bartels A, Zeki S. The neural basis of romantic love. Neuroreport. 2000. 11:3829–3834.41. Casey KL, Minoshima S, Morrow TJ, Koeppe RA. Comparison of human cerebral activation pattern during cutaneous warmth, heat pain, and deep cold pain. J Neurophysiol. 1996. 76:571–581.42. Schienle A, Stark R, Walter B, Blecker C, Ott U, Kirsch P, et al. The insula is not specifically involved in disgust processing: an fMRI study. Neuroreport. 2002. 13:2023–2026.43. George MS, Ketter TA, Parekh PI, Herscovitch P, Post RM. Gender differences in regional cerebral blood flow during transient self-induced sadness or happiness. Biol Psychiatry. 1996. 40:859–871.44. Davidson RJ, Irwin W. The functional neuroanatomy of emotion and affective style. Trends Cogn Sci. 1999. 3:11–21.45. Kross E, Egner T, Ochsner K, Hirsch J, Downey G. Neural dynamics of rejection sensitivity. J Cogn Neurosci. 2007. 19:945–956.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Neuro-Anatomical Evaluation of Human Suitability for Rural and Urban Environment by Using fMRI
- Functional Magnetic Resonance Imaging of the Brain: Principle and Practical Application
- An Understanding of Functional MR Imaging
- Functional Neuroanatomy in Depressed Patients with Sexual Dysfunction: Blood Oxygenation Level Dependent Functional MR Imaging
- Depression and the Frontal Lobe