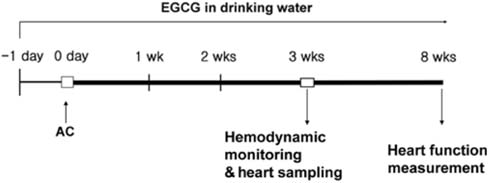
J Vet Sci.
2007 Jun;8(2):121-129. 10.4142/jvs.2007.8.2.121.
Epigallocatechin-3 gallate prevents cardiac hypertrophy induced by pressure overload in rats
- Affiliations
-
- 1Department of Pharmacology, College of Medicine, Chungbuk National University, Cheongju 361-763, Korea. hyahn@chungbuk.ac.kr
- 2Department of Pediatrics, College of Medicine, Chungbuk National University, Cheongju 361-763, Korea.
- KMID: 1089662
- DOI: http://doi.org/10.4142/jvs.2007.8.2.121
Abstract
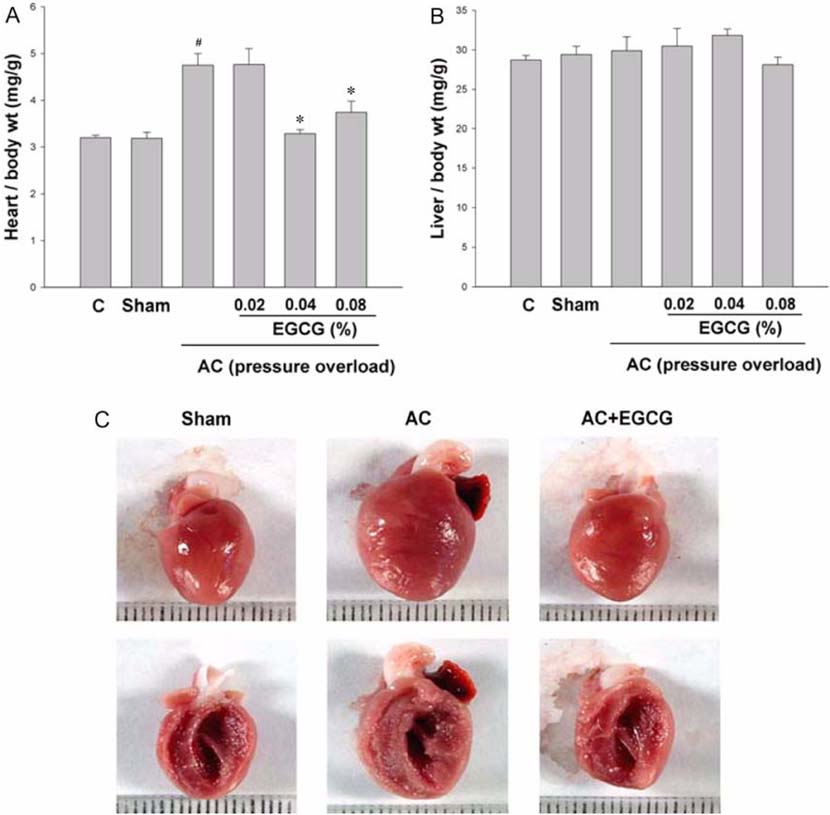
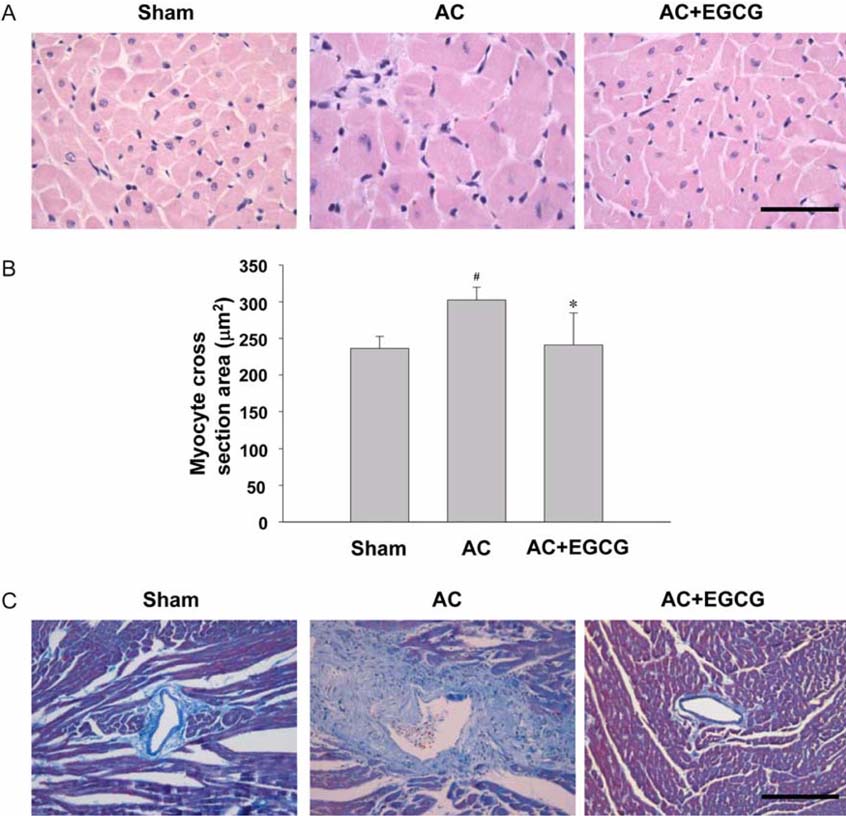
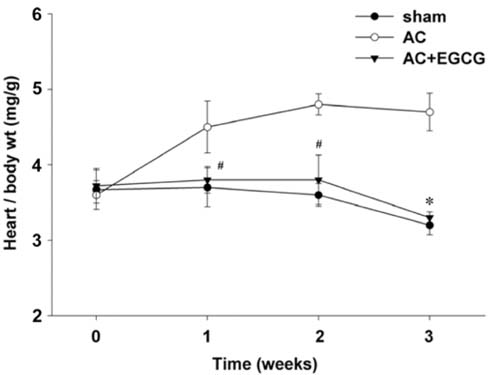
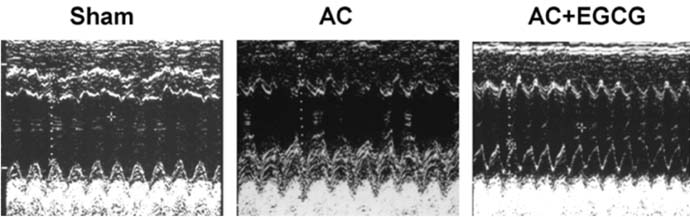
- Pressure overload diseases, such as valvular stenosis and systemic hypertension, manifest morphologically in patients as cardiac concentric hypertrophy. Prevention of cardiac remodeling due to increased pressure overload is important to reduce morbidity and mortality. Epigallocatechin-3 gallate (EGCG) is a major bioactive polyphenol present in green tea which has been found to be a nitric oxide-mediated vasorelaxant and to be cardioprotective in myocardial ischemia-reperfusion injury. Therefore, we investigated whether EGCG supplementation could reduce in vivo pressure overloadmediated cardiac hypertrophy. Cardiac hypertrophy was induced by suprarenal transverse abdominal aortic constriction (AC) in rats. Three weeks after AC surgery, heart to body weight ratio increased in the AC group by 34% compared to the sham group. EGCG administration suppressed the load-induced increase in heart weight by 69%. Attenuation of cardiac hypertrophy by EGCG was associated with attenuation of the increase in myocyte cell size and fibrosis induced by aortic constriction. Despite abolition of hypertrophy by EGCG, transstenotic pressure gradients did not change. Echocardiogram revealed that increased left ventricular systolic dimensions and deteriorated systolic function were relieved by EGCG. These results suggest that EGCG prevents the development of left ventricular concentric hypertrophy by pressure overload and may be a useful therapeutic modality to prevent cardiac remodeling in patients with pressure overload myocardial diseases.
Keyword
MeSH Terms
Figure
Reference
-
1. Ahn HY, Hadizadeh KR, Seul C, Yun YP, Vetter H, Sachinidis A. Epigallocathechin-3 gallate selectively inhibits the PDGF-BB-induced intracellular signaling transduction pathway in vascular smooth muscle cells and inhibits transformation of sis-transfected NIH 3T3 fibroblasts and human glioblastoma cells (A172). Mol Biol Cell. 1999. 10:1093–1104.
Article2. Baba HA, Iwai T, Bauer M, Irlbeck M, Schmid KW, Zimmer HG. Differential effects of angiotensin II receptor blockade on pressure-induced left ventricular hypertrophy and fibrosis in rats. J Mol Cell Cardiol. 1999. 31:445–455.
Article3. Chen L, Lee MJ, Li H, Yang CS. Absorption, distribution, elimination of tea polyphenols in rats. Drug Metab Dispos. 1997. 25:1045–1050.4. Chyu KY, Babbidge SM, Zhao X, Dandillaya R, Rietveld AG, Yano J, Dimayuga P, Cercek B, Shah PK. Differential effects of green tea-derived catechin on developing versus established atherosclerosis in apolipoprotein E-null mice. Circulation. 2004. 109:2448–2453.
Article5. Duarte J, Perez-Vizcaino F, Utrilla P, Jimenez J, Tamargo J, Zarzuelo A. Vasodilatory effects of flavonoids in rat aortic smooth muscle. Structure-activity relationships. Gen Pharmacol. 1993. 24:857–862.
Article6. Esposito G, Rapacciuolo A, Naga Prasad SV, Takaoka H, Thomas SA, Koch WJ, Rockman HA. Genetic alterations that inhibit in vivo pressure-overload hypertrophy prevent cardiac dysfunction despite increased wall stress. Circulation. 2002. 105:85–92.
Article7. Higuchi Y, Otsu K, Nishida K, Hirotani S, Nakayama H, Yamaguchi O, Matsumura Y, Ueno H, Tada M, Hori M. Involvement of reactive oxygen species-mediated NF-κB activation in TNF-α-induced cardiomyocyte hypertrophy. J Mol Cell Cardiol. 2002. 34:233–240.
Article8. Hill JA, Karimi M, Kutschke W, Davisson RL, Zimmerman K, Wang Z, Kerber RE, Weiss RM. Cardiac hypertrophy is not a required compensatory response to short-term pressure overload. Circulation. 2000. 101:2863–2869.
Article9. Hotta Y, Huang L, Muto T, Yajima M, Miyazeki K, Ishikawa N, Fukuzawa Y, Wakida Y, Tushima H, Ando H, Nonogaki T. Positive inotropic effect of purified green tea catechin derivative in guinea pig hearts: the measurements of cellular Ca2+ and nitric oxide release. Eur J Pharmacol. 2006. 552:123–130.
Article10. Jeong WS, Kim IW, Hu R, Kong AN. Modulatory properties of various natural chemopreventive agents on the activation of NF-κB signaling pathway. Pharm Res. 2004. 21:661–670.
Article11. Kao YH, Hiipakka RA, Liao S. Modulation of endocrine systems and food intake by green tea epigallocatechin gallate. Endocrinology. 2000. 141:980–987.
Article12. Li J, Li P, Feng X, Li Z, Hou R, Han C, Zhang Y. Effects of losartan on pressure overload-induced cardiac gene expression profiling in rats. Clin Exp Pharmacol Physiol. 2003. 30:827–832.
Article13. Li HL, Huang Y, Zhang CN, Liu G, Wei YS, Wang AB, Liu YQ, Hui RT, Wei C, Williams GM, Liu DP, Liang CC. Epigallocatechin-3 gallate inhibits cardiac hypertrophy through blocking reactive oxidative species-dependent and -independent signal pathways. Free Radic Biol Med. 2006. 40:1756–1775.
Article14. Lorenz M, Wessler S, Follmann E, Michaelis W, Dusterhoft T, Baumann G, Stangl K, Stangl V. A constituent of green tea, epigallocatechin-3-gallate, activates endothelial nitric oxide synthase by a phosphatidylinositol-3-OH-kinase-, cAMP-dependent protein kinase-, and Akt-dependent pathway and leads to endothelial-dependent vasorelaxation. J Biol Chem. 2004. 279:6190–6195.
Article15. Nagai K, Jiang MH, Hada J, Nagata T, Yajima Y, Yamamoto S, Nishizaki T. (-)-Epigallocatechin gallate protects against NO stress-induced neuronal damage after ischemia by acting as an anti-oxidant. Brain Res. 2002. 956:319–322.
Article16. Nakamura K, Fushimi K, Kouchi H, Mihara K, Miyazaki M, Ohe T, Namba M. Inhibitory effects of antioxidants on neonatal rat cardiac myocyte hypertrophy induced by tumor necrosis factor-α and angiotensin II. Circulation. 1998. 98:794–799.
Article17. Mosterd A, Hoes AW, de Bruyne MC, Deckers JW, Linker DT, Hofman A, Grobbee DE. Prevalence of heart failure and left ventricular dysfunction in the general population; The Rotterdam Study. Eur Heart J. 1999. 20:447–455.
Article18. Murray CJ, Lopez AD. Mortality by cause for eight regions of the world: Global Burden of Disease Study. Lancet. 1997. 349:1269–1276.
Article19. Pimentel DR, Amin JK, Xiao L, Miller T, Viereck J, Oliver-Krasinski J, Baliga R, Wang J, Siwik DA, Singh K, Pagano P, Colucci WS, Sawyer DB. Reactive oxygen species mediate amplitude-dependent hypertrophic and apoptotic responses to mechanical stretch in cardiac myocytes. Circ Res. 2001. 89:453–460.
Article20. Priyadarshi S, Valentine B, Han C, Fedorova OV, Bagrov AY, Liu J, Periyasamy SM, Kennedy D, Malhotra D, Xie Z, Shapiro JI. Effect of green tea extract on cardiac hypertrophy following 5/6 nephrectomy in the rat. Kidney Int. 2003. 63:1785–1790.
Article21. Ruwhof C, van der Laarse A. Mechanical stress-induced cardiac hypertrophy: mechanisms and signal transduction pathways. Cardiovasc Res. 2000. 47:23–37.
Article22. Siwik DA, Tzortzis JD, Pimental DR, Chang DL, Pagano PJ, Singh K, Sawyer DB, Colucci WS. Inhibition of copper-zinc superoxide dismutase induces cell growth, hypertrophic phenotype, and apoptosis in neonatal rat cardiac myocytes in vitro. Circ Res. 1999. 85:147–153.
Article23. Townsend PA, Scarabelli TM, Pasini E, Gitti G, Menegazzi M, Suzuki H, Knight RA, Latchman DS, Stephanou A. Epigallocatechin-3-gallate inhibits STAT-1 activation and protects cardiac myocytes from ischemia/reperfusion-induced apoptosis. FASEB J. 2004. 18:1621–1623.
Article24. Ventura HO, Malik FS, Mehra MR, Stapleton DD, Smart FW. Mechanisms of hypertension in cardiac transplantation and the role of cyclosporine. Curr Opin Cardiol. 1997. 12:375–381.
Article25. von Anrep G. On the part played by the suprarenals in the normal vascular reactions of the body. J Physiol. 1912. 45:307–317.
Article26. Yamaguchi O, Higuchi Y, Hirotani S, Kashiwase K, Nakayama H, Hikoso S, Takeda T, Watanabe T, Asahi M, Taniike M, Matsumura Y, Tsujimoto I, Hongo K, Kusakari Y, Kurihara S, Nishida K, Ichijo H, Hori M, Otsu K. Targeted deletion of apoptosis signal-regulating kinase 1 attenuates left ventricular remodeling. Proc Natl Acad Sci USA. 2003. 100:15883–15888.
Article27. Yue TL, Gu JL, Wang C, Reith AD, Lee JC, Mirabile RC, Kreutz R, Wang Y, Maleeff B, Parsons AA, Ohlstein EH. Extracellular signal-regulated kinase plays an essential role in hypertrophic agonists, endothelin-1 and phenylephrine-induced cardiomyocyte hypertrophy. J Biol Chem. 2000. 275:37895–37901.
Article28. Zheng Y, Song HJ, Kim CH, Kim HS, Kim EG, Sachinidis A, Ahn HY. Inhibitory effect of epigallocatechin 3-O-gallate on vascular smooth muscle cell hypertrophy induced by angiotensin II. J Cardiovasc Pharmacol. 2004. 43:200–208.
Article29. Zou Y, Komuro I, Yamazaki T, Kudoh S, Aikawa R, Zhu W, Shiojima I, Hiroi Y, Tobe K, Kadowaki T, Yazaki Y. Cell type-specific angiotensin II-evoked signal transduction pathways: critical roles of Gβγ subunit, Src family, and Ras in cardiac fibroblasts. Circ Res. 1998. 82:337–345.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Comparing the Effects of Carvedilol Enantiomers on Regression of Established Cardiac Hypertrophy Induced by Pressure Overload
- EGCG Blocked Phenylephrin-Induced Hypertrophy in H9C2 Cardiomyocytes, by Activating AMPK-Dependent Pathway
- Transactivation of peroxisome proliferator-activated receptor alpha by green tea extracts
- The Effects of (-)-Epigallocatechin Gallate on Rat Hippocampal Organotypic Slice Cultures Treated with the 1-42 beta-amyloid Protein
- Electron Microscopic Study of Enalapril Effect on Left Ventricular Hypertrophy in Spontaneously Hypertensive Rat