Yonsei Med J.
2008 Apr;49(2):311-321. 10.3349/ymj.2008.49.2.311.
Alteration of Expression of Ca(2+) Signaling Proteins and Adaptation of Ca(2+) Signaling in SERCA2(+/-) Mouse Parotid Acini
- Affiliations
-
- 1Department of Oral Medicine, Oral Science Research Center, Center for Natural Defense System, BK21 Project, Yonsei University College of Dentistry, Seoul, Korea. dmshin@yuhs.ac
- 2Department of Oral Biology, Oral Science Research Center, Center for Natural Defense System, BK21 Project, Yonsei University College of Dentistry, Seoul, Korea.
- KMID: 1084506
- DOI: http://doi.org/10.3349/ymj.2008.49.2.311
Abstract
- PURPOSE
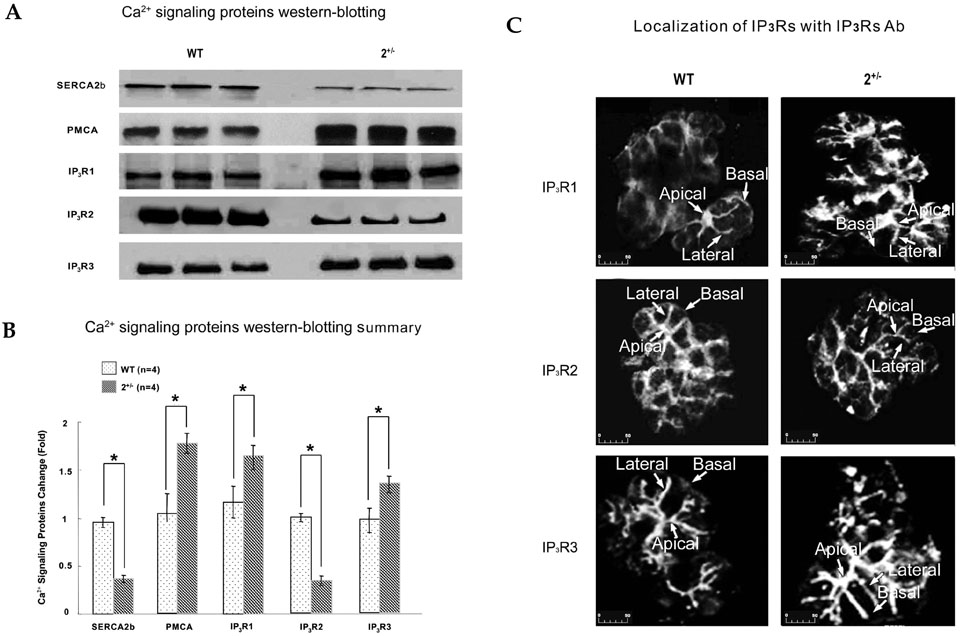
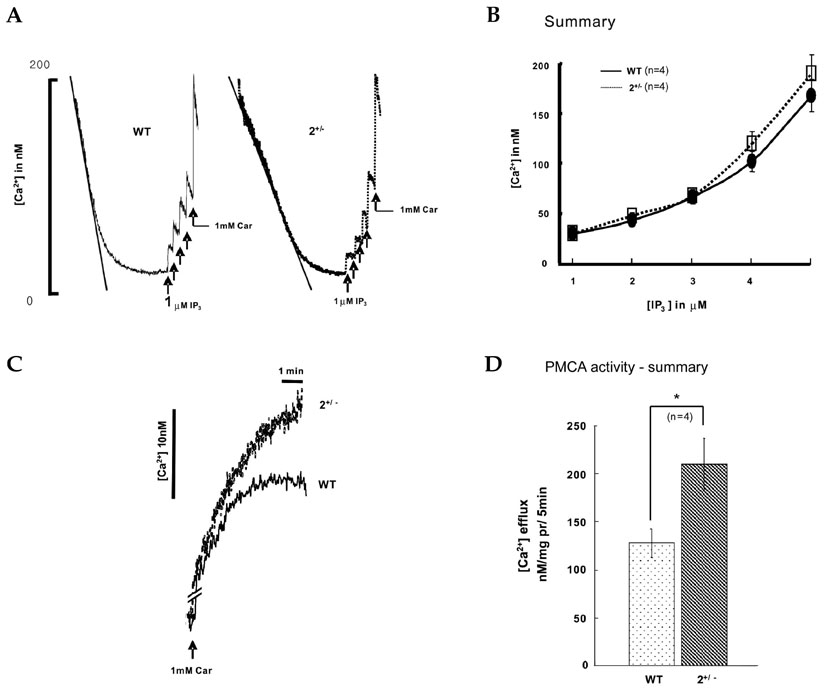
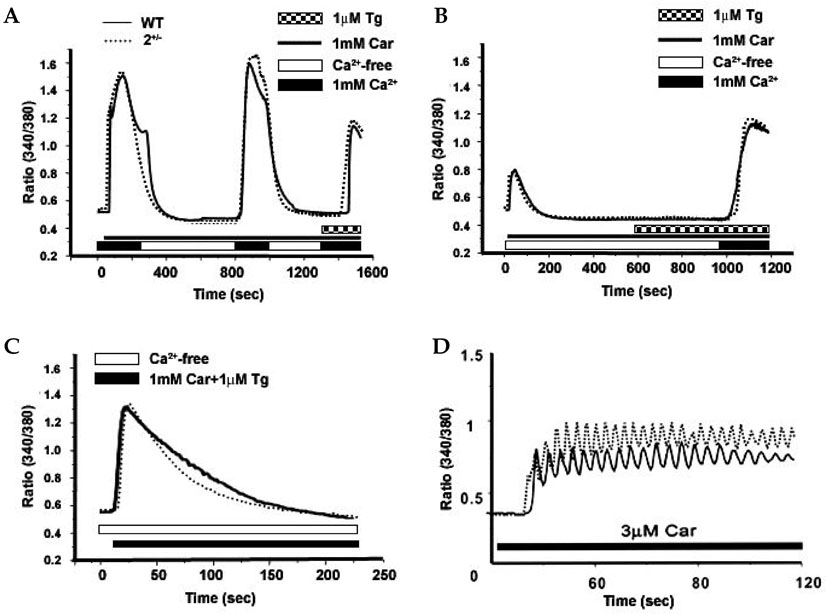
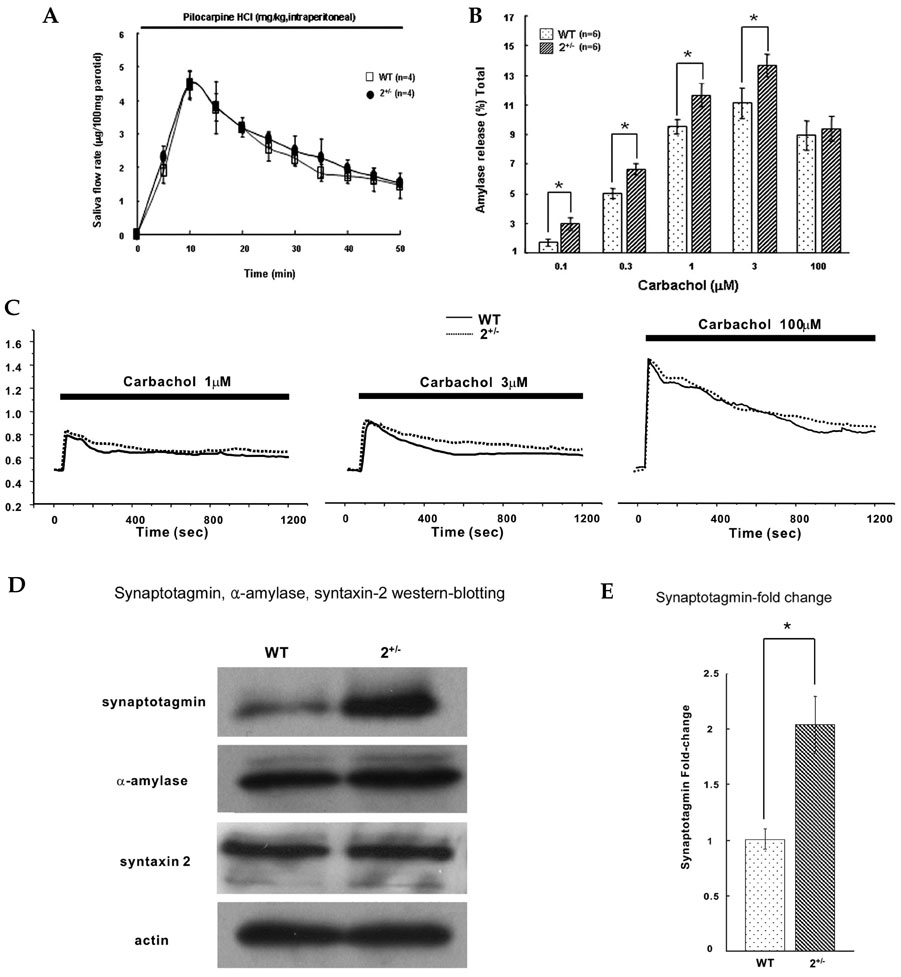
The sarco/endoplasmic reticulum Ca(2+)-ATPase (SERCA), encoded by ATP2A2, is an essential component for G-protein coupled receptor (GPCR)-dependent Ca(2+) signaling. However, whether the changes in Ca(2+) signaling and Ca(2+) signaling proteins in parotid acinar cells are affected by a partial loss of SERCA2 are not known. MATERIALS AND METHODS: In SERCA2(+/-) mouse parotid gland acinar cells, Ca(2+) signaling, expression levels of Ca(2+) signaling proteins, and amylase secretion were investigated. RESULTS: SERCA2(+/-) mice showed decreased SERCA2 expression and an upregulation of the plasma membrane Ca(2+) ATPase. A partial loss of SERCA2 changed the expression level of 1, 4, 5-tris-inositolphosphate receptors (IP(3)Rs), but the localization and activities of IP3Rs were not altered. In SERCA2(+/-) mice, muscarinic stimulation resulted in greater amylase release, and the expression of synaptotagmin was increased compared to wild type mice. CONCLUSION: These results suggest that a partial loss of SERCA2 affects the expression and activity of Ca(2+) signaling proteins in the parotid gland acini, however, overall Ca(2+) signaling is unchanged.
Keyword
MeSH Terms
-
Amylases/metabolism
Animals
Blotting, Western
Calcium/metabolism
Calcium Signaling/drug effects/genetics/*physiology
Carbachol/pharmacology
Immunohistochemistry
Inositol 1,4,5-Trisphosphate Receptors/metabolism
Mice
Mice, Knockout
Parotid Gland/*metabolism
Sarcoplasmic Reticulum Calcium-Transporting ATPases/genetics/*metabolism
Signal Transduction/drug effects/genetics/physiology
Figure
Reference
-
1. Turner RJ, Sugiya H. Understanding salivary fluid and protein secretion. Oral Dis. 2002. 8:3–11.
Article2. Takuma T, Ichida T. Evidence for the involvement of protein phosphorylation in cyclic AMP-mediated amylase exocytosis from parotid acinar cells. FEBS Lett. 1994. 340:29–33.
Article3. Sugiya H, Furuyama S. The activation of Ca2+ mobilizing receptors in salivary gland. Biomed Res. 1989. 10:111–121.4. Berridge MJ, Bootman MD, Roderick HL. Calcium signalling: dynamics, homeostasis and remodelling. Nat Rev Mol Cell Biol. 2003. 4:517–529.
Article5. Kiselyov K, Shin DM, Muallem S. Signalling specificity in GPCR-dependent Ca2+ signalling. Cell Signal. 2003. 15:243–253.6. East JM. Sarco(endo)plasmic reticulum calcium pumps: recent advances in our understanding of structure/function and biology (review). Mol Membr Biol. 2000. 17:189–200.
Article7. Bolotina VM. Store-operated channels: diversity and activation mechanisms. Sci STKE. 2004. 243:pe34.
Article8. Lee MG, Xu X, Zeng W, Diaz J, Wojcikiewicz RJ, Kuo TH, et al. Polarized expression of Ca2+ channels in pancreatic and salivary gland cells. Correlation with initiation and propagation of [Ca2+]i waves. J Biol Chem. 1997. 272:15765–15770.
Article9. Anwar A, Taimor G, Korkususz H, Schreckenberg R, Berndt T, Abdallah Y, et al. PKC- independent signal transduction pathways increase SERCA2 expression in adult rat cardiomyocytes. J Mol Cell Cardiol. 2005. 39:911–919.
Article10. Verboomen H, Wuytack F, Van den Bosch L, Mertens L, Casteels R. The functional importance of the extreme C-terminal tail in the gene 2 organellar Ca2+-transport ATPase (SERCA 2a/b). Biochem J. 1994. 303:979–984.
Article11. Periasamy M, Reed TD, Liu LH, Ji Y, Loukianov E, Paul RJ, et al. Impaired cardiac performance in heterozygous mice with a null mutation in the sarco(endo)plasmic reticulum Ca2+-ATPase isoform 2 (SERCA2) gene. J Biol Chem. 1999. 274:2556–2562.
Article12. Prasad V, Okunade GW, Miller ML, Shull GE. Phenotypes of SERCA and PMCA knockout mice. Biochem Biophys Res Commun. 2004. 322:1192–1203.
Article13. Zhao XS, Shin DM, Liu LH, Shull GE, Muallem S. Plasticity and adaptation of Ca2+ signaling and Ca2+- dependent exocytosis in SERCA2+/- mice. EMBO J. 2001. 20:2680–2689.
Article14. Ruiz-Perez VL, Carter SA, Healy E, Todd C, Rees JL, Steijlen PM, et al. ATP2A2 mutations in Darier's disease: variant cutaneous phenotypes are associated with missense mutations, but neuropsychiatric features are independent of mutation class. Hum Mol Genet. 1999. 9:1621–1630.15. Bernfeld P. Enzymes of starch degradation and synthesis. Adv Enzymol Relat Subj Biochem. 1951. 12:379–428.
Article16. Shin DM, Luo X, Wilkie TM, Miller LJ, Peck AB, Humphreys-Beher MG, et al. Polarized expression of G protein-coupled receptors and an all-or-none discharge of Ca2+ pools at initiation sites of [Ca2+]i waves in polarized exocrine cells. J Biol Chem. 2001. 276:44146–44156.
Article17. Zhang BX, Muallem S. Feedback inhibition of Ca2+ release by Ca2+ is the underlying mechanism of agonist-evoked intracellular Ca2+ oscillations in pancreatic acinar cells. J Biol Chem. 1992. 267:24387–24393.
Article18. Ji Y, Lalli MJ, Babu GJ, Xu Y, Kirkpatrick DL, Liu LH, et al. Disruption of a single copy of the SERCA2 gene results in altered Ca2+ homeostasis and cardiomyocyte function. J Biol Chem. 2000. 275:38073–38080.
Article19. Chapman ER. Synaptotagmin: A Ca2+ sensor that triggers exocytosis? Nat Rev Mal Cell Biol. 2002. 3:498–508.20. Foskett JK, Roifman CM, Wong D. Activation of calcium oscillations by thapsigargin in parotid acinar cells. J Biol Chem. 1991. 266:2778–2782.
Article21. Thorn P, Lawrie AM, Smith PM, Gallacher DV, Petersen OH. Ca2+ oscillations in pancreatic acinar cells: spatiotemporal relationships and functional implications. Cell Calcium. 1993. 10:746–757.22. Newton CL, Mignery GA, Südhof TC. Co-expression in vertebrate tissues and cell lines of multiple inositol 1,4,5-trisphosphate (InsP3) receptors with distinct affinities for InsP3. J Biol Chem. 1994. 269:28613–28619.
Article23. Hagar RE, Burgstahler AD, Nathanson MH, Ehrlich BE. Type III InsP3 receptor channel stays open in the presence of increased calcium. Nature. 1998. 396:81–84.
Article24. Hattori M, Suzuki AZ, Higo T, Miyauchi H, Michikawa T, Nakamura T, et al. Distinct roles of inositol 1,4,5-trisphosphate receptor types 1 and 3 in Ca2+ signaling. J Biol Chem. 2004. 279:11967–11975.
Article25. Shin DM, Zhao XS, Zeng W, Mozhayeva M, Muallem S. The mammalian Sec6/8 complex interacts with Ca2+ signaling complexes and regulates their activity. J Cell Biol. 2000. 150:1101–1112.
Article26. Monkawa T, Miyawaki A, Sugiyama T, Yoneshima H, Yamamoto-Hino M, Furuichi T, et al. Heterotetrameric complex formation of inositol 1,4,5-trisphosphate receptor subunits. J Biol Chem. 1995. 270:14700–14704.
Article27. Simon VR, Moran MF. SERCA activity is required for timely progression through G1/S. Cell Prolif. 2001. 34:15–30.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Homer2 regulates amylase secretion via physiological calcium oscillations in mouse parotid gland acinar cells
- Regulation of the expression and function of TRPCs and Orai1 by Homer2 in mouse pancreatic acinar cells
- Afatinib Mediates Autophagic Degradation of ORAI1, STIM1, and SERCA2, Which Inhibits Proliferation of Non–Small Cell Lung Cancer Cells
- Initiation Site of Ca2+ Entry Evoked by Endoplasmic Reticulum Ca2+ Depletion in Mouse Parotid and Pancreatic Acinar Cells
- Immunohistochemical Localizaion of Carbonic Anhydrase Isozymes IV and IX in Rat Salivary Gland