Yonsei Med J.
2010 May;51(3):438-447. 10.3349/ymj.2010.51.3.438.
Knockdown of Moesin Expression Accelerates Cellular Senescence of Human Dermal Microvascular Endothelial Cells
- Affiliations
-
- 1Department of Dermatology and Cutaneous Biology Research Institute, Yonsei University College of Medicine, Seoul, Korea. kwanglee@yuhs.ac
- KMID: 1074998
- DOI: http://doi.org/10.3349/ymj.2010.51.3.438
Abstract
- PURPOSE
Endothelial cells maintain the homeostasis of blood, which consists of plasma and cellular components, and regulate the interaction between blood and the surrounding tissues. They also have essential roles in vascular permeability, the circulation, coagulation, inflammation, wound healing, and tissue growth. The senescence of endothelial cells is closely related to the aging of the adjacent tissues and to age-related vascular disease. Recently, the expression of moesin was found to be decreased in elderly human dermal microvascular endothelial cells (HDMECs), and an association between moesin and senescence has been suggested. This study examined the functional role of moesin in cellular senescence.
MATERIALS AND METHODS
To study the effects of decreased moesin expression on cellular senescence and metabolism, HDMECs were transfected with short hairpin-RNA (shRNA) lentivirus to silence moesin gene expression. In addition, specimens from young and old human skin were stained with anti-moesin and anti-p16 antibodies as an in vivo study.
RESULTS
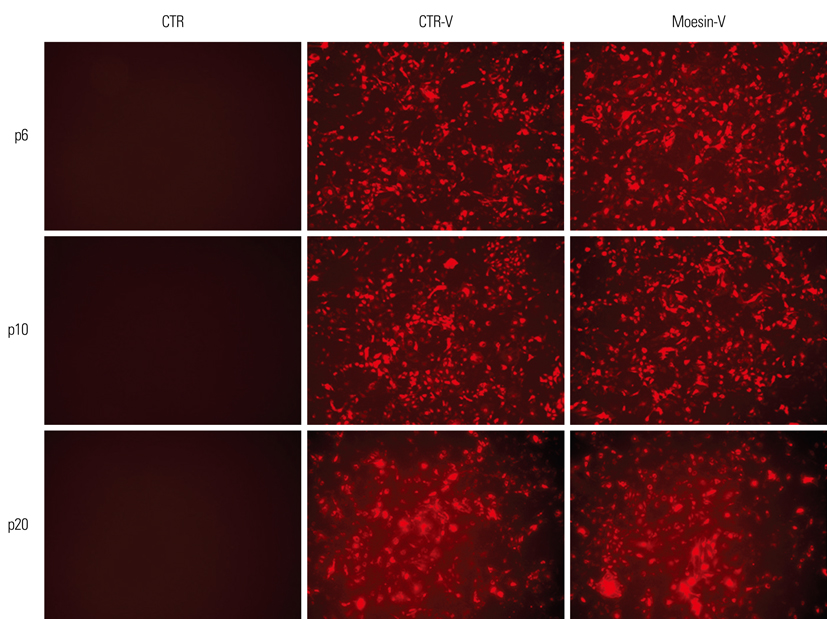
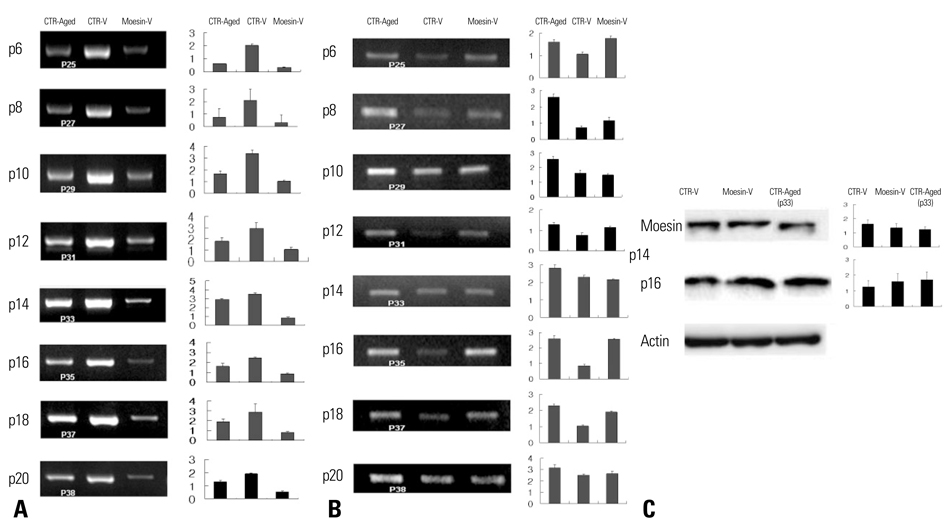
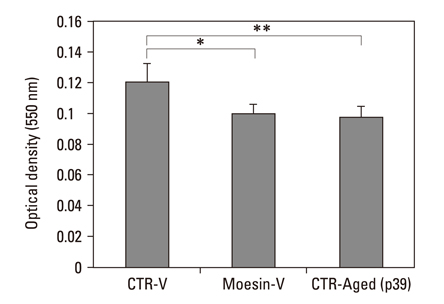
Using shRNAl-entivirus, moesin knock-down HDMECs developed characteristics associated with aging and expressed senescence associated-beta-galactosidase during early passages. They also showed increased p16 expression, decreased metabolic activity, and cell growth retardation. Human skin tissue from elderly persons showed decreased moesin expression and increased p16 expression.
CONCLUSION
These findings suggest that there is a functional association between moesin expression and cellular senescence. Further study of the functional mechanism of moesin in the cytoskeleton and cellular senescence is needed. In addition, this study provides a useful model for developing anti-aging treatments.
Keyword
MeSH Terms
-
Aged, 80 and over
Antigens, CD31/metabolism
Blotting, Western
Cell Aging/genetics/*physiology
Cell Line
Child
Endothelial Cells/*cytology/*metabolism
Humans
Immunohistochemistry
Microfilament Proteins/genetics/*physiology
Microscopy, Phase-Contrast
Microvessels/*cytology
RNA, Small Interfering/genetics/physiology
Reverse Transcriptase Polymerase Chain Reaction
Skin/*blood supply
Figure
Reference
-
1. Ben-Porath I, Weinberg RA. The signals and pathways activating cellular senescence. Int J Biochem Cell Biol. 2005. 37:961–976.
Article2. Kujoth GC, Hiona A, Pugh TD, Someya S, Panzer K, Wohlgemuth SE, et al. Mitochondrial DNA mutations, oxidative stress, and apoptosis in mammalian aging. Science. 2005. 309:481–484.
Article3. Shelton DN, Chang E, Whittier PS, Choi D, Funk WD. Microarray analysis of replicative senescence. Curr Biol. 1999. 9:939–945.
Article4. Comi P, Chiaramonte R, Maier JA. Senescence-dependent regulation of type 1 plasminogen activator inhibitor in human vascular endothelial cells. Exp Cell Res. 1995. 219:304–308.
Article5. Tang J, Gordon GM, Nickoloff BJ, Foreman KE. The helix-loophelix protein id-1 delays onset of replicative senescence in human endothelial cells. Lab Invest. 2002. 82:1073–1079.
Article6. Wagner M, Hampel B, Bernhard D, Hala M, Zwerschke W, Jansen-Dürr P. Replicative senescence of human endothelial cells in vitro involves G1 arrest, polyploidization and senescence-associated apoptosis. Exp Gerontol. 2001. 36:1327–1347.
Article7. Pearson JD. Normal endothelial cell function. Lupus. 2000. 9:183–188.
Article8. Cines DB, Pollak ES, Buck CA, Loscalzo J, Zimmerman GA, McEver RP, et al. Endothelial cells in physiology and in the pathophysiology of vascular disorders. Blood. 1998. 91:3527–3561.9. Hasdai D, Sangiorgi G, Spagnoli LG, Simari RD, Holmes DR Jr, Kwon HM, et al. Coronary artery apoptosis in experimental hypercholesterolemia. Atherosclerosis. 1999. 142:317–325.
Article10. Haunstetter A, Izumo S. Apoptosis: basic mechanisms and implications for cardiovascular disease. Circ Res. 1998. 82:1111–1129.11. Singhal PC, Gibbons N, Franki N, Reddy K, Sharma P, Mattana J, et al. Simulated glomerular hypertension promotes mesangial cell apoptosis and expression of cathepsin-B and SGP-2. J Investig Med. 1998. 46:42–50.12. Vescovo G, Zennaro R, Sandri M, Carraro U, Leprotti C, Ceconi C, et al. Apoptosis of skeletal muscle myofibers and interstitial cells in experimental heart failure. J Mol Cell Cardiol. 1998. 30:2449–2459.13. Galley HF, Howdle PD, Walker BE, Webster NR. The effects of intravenous antioxidants in patients with septic shock. Free Radic Biol Med. 1997. 23:768–774.14. Mizutani M, Kern TS, Lorenzi M. Accelerated death of retinal microvascular cells in human and experimental diabetic retinopathy. J Clin Invest. 1996. 97:2883–2890.
Article15. Sgonc R, Gruschwitz MS, Dietrich H, Recheis H, Gershwin ME, Wick G. Endothelial cell apoptosis is a primary pathogenetic event underlying skin lesions in avian and human scleroderma. J Clin Invest. 1996. 98:785–792.
Article16. Dang CT, Magid MS, Weksler B, Chadburn A, Laurence J. Enhanced endothelial cell apoptosis in splenic tissues of patients with thrombotic thrombocytopenic purpura. Blood. 1999. 93:1264–1270.
Article17. Lai KN, Leung JC, Lai KB, Lai CK. Effect of anti-DNA autoantibodies on the gene expression of interleukin 8, transforming growth factor-beta, and nitric oxide synthase in cultured endothelial cells. Scand J Rheumatol. 1997. 26:461–467.18. Kebers F, Lewalle JM, Desreux J, Munaut C, Devy L, Foidart JM, et al. Induction of endothelial cell apoptosis by solid tumor cells. Exp Cell Res. 1998. 240:197–205.19. Ha MK, Soo Cho J, Baik OR, Lee KH, Koo HS, Chung KY. Caenorhabditis elegans as a screening tool for the endothelial cell-derived putative aging-related proteins detected by proteomic analysis. Proteomics. 2006. 6:3339–3351.20. Lee JH, Chung KY, Bang D, Lee KH. Searching for aging-related proteins in human dermal microvascular endothelial cells treated with anti-aging agents. Proteomics. 2006. 6:1351–1361.
Article21. Chishti AH, Kim AC, Marfatia SM, Lutchman M, Hanspal M, Jindal H, et al. The FERM domain: a unique module involved in the linkage of cytoplasmic proteins to the membrane. Trends Biochem Sci. 1998. 23:281–282.22. Bretscher A, Edwards K, Fehon RG. ERM proteins and merlin: integrators at the cell cortex. Nat Rev Mol Cell Biol. 2002. 3:586–599.
Article23. Fiévet B, Louvard D, Arpin M. ERM proteins in epithelial cell organization and functions. Biochim Biophys Acta. 2007. 1773:653–660.24. Tsukita S, Yonemura S. ERM (ezrin/radixin/moesin) family: from cytoskeleton to signal transduction. Curr Opin Cell Biol. 1997. 9:70–75.25. Miller KG. A role for moesin in polarity. Trends Cell Biol. 2003. 13:165–168.
Article26. Polesello C, Payre F. Small is beautiful: what flies tell us about ERM protein function in development. Trends Cell Biol. 2004. 14:294–302.
Article27. Charrin S, Alcover A. Role of ERM (ezrin-radixin-moesin) proteins in T lymphocyte polarization, immune synapse formation and in T cell receptor-mediated signaling. Front Biosci. 2006. 11:1987–1997.
Article28. Ivetic A, Ridley AJ. Ezrin/radixin/moesin proteins and Rho GTPase signalling in leucocytes. Immunology. 2004. 112:165–176.
Article29. Simoncini T, Scorticati C, Mannella P, Fadiel A, Giretti MS, Fu XD, et al. Estrogen receptor alpha interacts with Galpha13 to drive actin remodeling and endothelial cell migration via the RhoA/Rho kinase/moesin pathway. Mol Endocrinol. 2006. 20:1756–1771.30. Brown LM, Helmke SM, Hunsucker SW, Netea-Maier RT, Chiang SA, Heinz DE, et al. Quantitative and qualitative differences in protein expression between papillary thyroid carcinoma and normal thyroid tissue. Mol Carcinog. 2006. 45:613–626.
Article31. Craven RA, Stanley AJ, Hanrahan S, Dods J, Unwin R, Totty N, et al. Proteomic analysis of primary cell lines identifies protein changes present in renal cell carcinoma. Proteomics. 2006. 6:2853–2864.32. Torer N, Kayaselcuk F, Nursal TZ, Yildirim S, Tarim A, Nòyan T, et al. Adhesion molecules as prognostic markers in pancreatic adenocarcinoma. J Surg Oncol. 2007. 96:419–423.
Article33. Fais S, De Milito A, Lozupone F. The role of FAS to ezrin association in FAS-mediated apoptosis. Apoptosis. 2005. 10:941–947.
Article34. Krawetz R, MacKenzie MJ, Sun Q, Walton PA, Kelly GM. Galpha13 activation rescues moesin-depletion induced apoptosis in F9 teratocarcinoma cells. Exp Cell Res. 2006. 312:3224–3240.35. Hayflick L, Moorhead PS. The serial cultivation of human diploid cell strains. Exp Cell Res. 1961. 25:585–621.
Article36. Weinman EJ, Steplock D, Donowitz M, Shenolikar S. NHERF associations with sodium-hydrogen exchanger isoform 3 (NHE3) and ezrin are essential for cAMP-mediated phosphorylation and inhibition of NHE3. Biochemistry. 2000. 39:6123–6129.37. Shiue H, Musch MW, Wang Y, Chang EB, Turner JR. Akt2 phosphorylates ezrin to trigger NHE3 translocation and activation. J Biol Chem. 2005. 280:1688–1695.
Article38. Chorna-Ornan I, Tzarfaty V, Ankri-Eliahoo G, Joel-Almagor T, Meyer NE, Huber A, et al. Light-regulated interaction of Dmoesin with TRP and TRPL channels is required for maintenance of photoreceptors. J Cell Biol. 2005. 171:143–152.39. Erwig LP, McPhilips KA, Wynes MW, Ivetic A, Ridley AJ, Henson PM. Differential regulation of phagosome maturation in macrophages and dendritic cells mediated by Rho GTPases and ezrin-radixin-moesin (ERM) proteins. Proc Natl Acad Sci U S A. 2006. 103:12825–12830.40. Doulet N, Donnadieu E, Laran-Chich MP, Niedergang F, Nassif X, Couraud PO, et al. Neisseria meningitidis infection of human endothelial cells interferes with leukocyte transmigration by preventing the formation of endothelial docking structures. J Cell Biol. 2006. 173:627–637.
Article41. Elbashir SM, Harborth J, Lendeckel W, Yalcin A, Weber K, Tuschl T. Duplexes of 21-nucleotide RNAs mediate RNA interference in cultured mammalian cells. Nature. 2001. 411:494–498.42. Brummelkamp TR, Bernards R, Agami R. A system for stable expression of short interfering RNAs in mammalian cells. Science. 2002. 296:550–553.43. Dimri GP, Lee X, Basile G, Acosta M, Scott G, Roskelley C, et al. A biomarker that identifies senescent human cells in culture and in aging skin in vivo. Proc Natl Acad Sci U S A. 1995. 92:9363–9367.
Article44. van der Loo B, Fenton MJ, Erusalimsky JD. Cytochemical detection of a senescence-associated beta-galactosidase in endothelial and smooth muscle cells from human and rabbit blood vessels. Exp Cell Res. 1998. 241:309–315.
Article45. Severino J, Allen RG, Balin S, Balin A, Cristofalo VJ. Is beta-galactosidase staining a marker of senescence in vitro and in vivo? Exp Cell Res. 2000. 257:162–171.46. Kiyokawa H. Senescence and cell cycle control. Results Probl Cell Differ. 2006. 42:257–270.
Article47. Lou Z, Chen J. Cellular senescence and DNA repair. Exp Cell Res. 2006. 312:2641–2646.48. Jacobs JJ, de Lange T. Significant role for p16INK4a in p53-independent telomere-directed senescence. Curr Biol. 2004. 14:2302–2308.
Article49. Iwasa H, Han J, Ishikawa F. Mitogen-activated protein kinase p38 defines the common senescence-signalling pathway. Genes Cells. 2003. 8:131–144.
Article50. Singh KK. Mitochondria damage checkpoint, aging, and cancer. Ann N Y Acad Sci. 2006. 1067:182–190.
Article51. Hara E, Smith R, Parry D, Tahara H, Stone S, Peters G. Regulation of p16CDKN2 expression and its implications for cell immortalization and senescence. Mol Cell Biol. 1996. 16:859–867.52. Alcorta DA, Xiong Y, Phelps D, Hannon G, Beach D, Barrett JC. Involvement of the cyclin-dependent kinase inhibitor p16 (INK4a) in replicative senescence of normal human fibroblasts. Proc Natl Acad Sci U S A. 1996. 93:13742–13747.
Article53. Ressler S, Bartkova J, Niederegger H, Bartek J, Scharffetter-Kochanek K, Jansen-Durr P, et al. p16INK4A is a robust in vivo biomarker of cellular aging in human skin. Aging Cell. 2006. 5:379–389.54. Verrills NM, Liem NL, Liaw TY, Hood BD, Lock RB, Kavallaris M. Proteomic analysis reveals a novel role for the actin cytoskeleton in vincristine resistant childhood leukemia--an in vivo study. Proteomics. 2006. 6:1681–1694.55. Madan R, Brandwein-Gensler M, Schlecht NF, Elias K, Gorbovitsky E, Belbin TJ, et al. Differential tissue and subcellular expressionof ERM proteins in normal and malignant tissues: cytoplasmic ezrin expression has prognostic signficance for head and neck squamous cell carcinoma. Head Neck. 2006. 28:1018–1027.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Nervonic Acid Inhibits Replicative Senescence of Human Wharton’s Jelly-Derived Mesenchymal Stem Cells
- Regulation of V-cadherin Expression on Human Dermal Microvascular Endothelial Cells
- Autophagy May Mediate Cellular Senescence by Nicotine Stimulation in Gingival Fibroblasts
- Enhanced Viral Replication by Cellular Replicative Senescence
- A Novel Role of Hyaluronic Acid and Proteoglycan Link Protein 1 (HAPLN1) in Delaying Vascular Endothelial Cell Senescence