Yonsei Med J.
2011 Nov;52(6):999-1007. 10.3349/ymj.2011.52.6.999.
Rapid Isolation of Adipose Tissue-Derived Stem Cells by the Storage of Lipoaspirates
- Affiliations
-
- 1Cell Therapy and Tissue Engineering Center, Wonju College of Medicine, Yonsei University, Wonju, Korea.
- 2Department of Hematology-Oncology, Wonju College of Medicine, Yonsei University, Wonju, Korea. khsmd@unitel.co.kr
- 3Biomedical Research Institute, Lifeliver. Co., Ltd., Suwon, Korea.
- 4Dr. Park's Aesthetic Clinic, Seoul, Korea.
- KMID: 1058822
- DOI: http://doi.org/10.3349/ymj.2011.52.6.999
Abstract
- PURPOSE
This study examined a rapid isolation method decreasing the time and cost of the clinical application of adipose tissue-derived stem cells (ASCs).
MATERIALS AND METHODS
Aliquots (10 g) of the lipoaspirates were stored at 4degrees C without supplying oxygen or nutrients. At the indicated time points, the yield of mononuclear cells was evaluated and the stem cell population was counted by colony forming unit-fibroblast assays. Cell surface markers, stem cell-related transcription factors, and differentiation potentials of ASCs were analyzed.
RESULTS
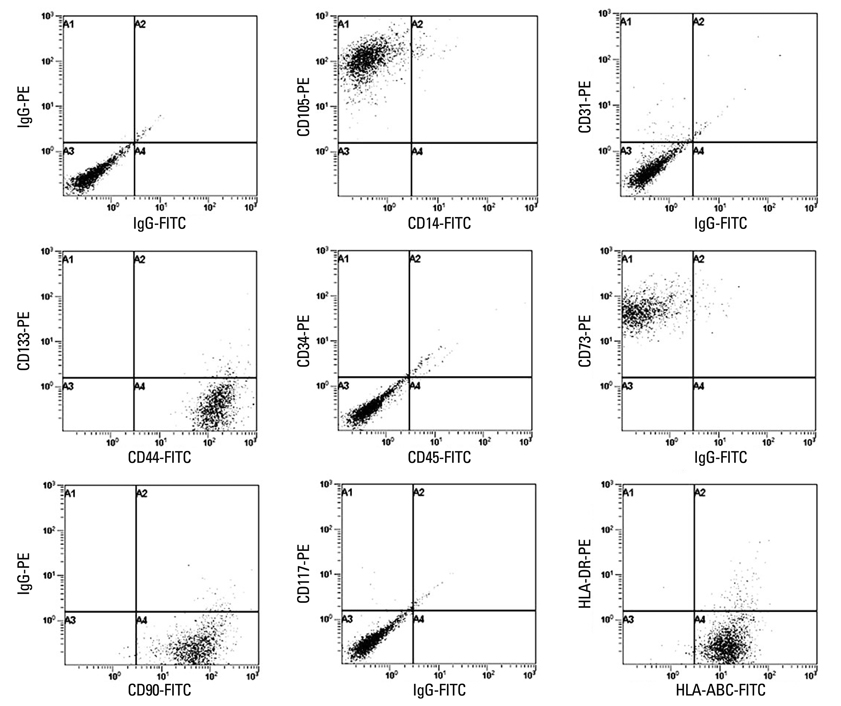
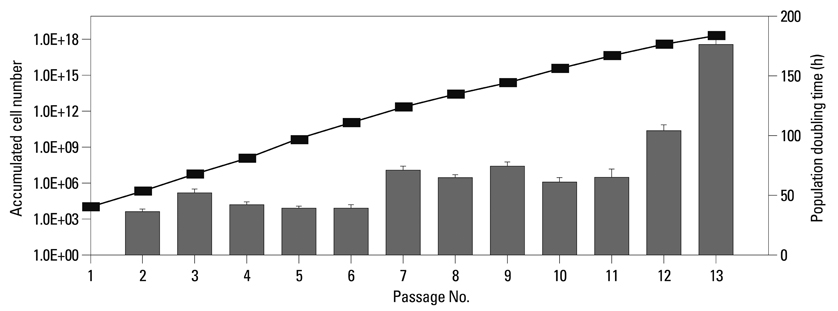
When the lipoaspirates were stored at 4degrees C, the total yield of mononuclear cells decreased, but the stem cell population was enriched. These ASCs expressed CD44, CD73, CD90, CD105, and HLA-ABC but not CD14, CD31, CD34, CD45, CD117, CD133, and HLA-DR. The number of ASCs increased 1x1014 fold for 120 days. ASCs differentiated into osteoblasts, adipocytes, muscle cells, or neuronal cells.
CONCLUSION
ASCs isolated from lipoaspirates and stored for 24 hours at 4degrees C have similar properties to ASCs isolated from fresh lipoaspirates. Our results suggest that ASCs can be isolated with high frequency by optimal storage at 4degrees C for 24 hours, and those ASCs are highly proliferative and multipotent, similar to ASCs isolated from fresh lipoaspirates. These ASCs can be useful for clinical application because they are time- and cost-efficient, and these cells maintain their stemness for a long time, like ASCs isolated from fresh lipoaspirates.
MeSH Terms
-
5'-Nucleotidase/metabolism
Adipose Tissue/*cytology
Adult
Antigens, CD/metabolism
Antigens, CD44/metabolism
Antigens, Thy-1/metabolism
Cell Differentiation/physiology
Cells, Cultured
Female
Humans
Immunoblotting
Immunohistochemistry
Immunophenotyping
Mesenchymal Stem Cells/metabolism
Muscle Development/genetics/physiology
Osteogenesis/genetics/physiology
Receptors, Cell Surface/metabolism
Reverse Transcriptase Polymerase Chain Reaction
Stem Cells/*cytology/metabolism
Young Adult
Figure
Reference
-
1. Colter DC, Class R, DiGirolamo CM, Prockop DJ. Rapid expansion of recycling stem cells in cultures of plastic-adherent cells from human bone marrow. Proc Natl Acad Sci U S A. 2000. 97:3213–3218.
Article2. Sekiya I, Larson BL, Smith JR, Pochampally R, Cui JG, Prockop DJ. Expansion of human adult stem cells from bone marrow stroma: conditions that maximize the yields of early progenitors and evaluate their quality. Stem Cells. 2002. 20:530–541.
Article3. Haynesworth SE, Goshima J, Goldberg VM, Caplan AI. Characterization of cells with osteogenic potential from human marrow. Bone. 1992. 13:81–88.
Article4. Pittenger MF, Mackay AM, Beck SC, Jaiswal RK, Douglas R, Mosca JD, et al. Multilineage potential of adult human mesenchymal stem cells. Science. 1999. 284:143–147.
Article5. Prockop DJ. Marrow stromal cells as stem cells for nonhematopoietic tissues. Science. 1997. 276:71–74.
Article6. Friedenstein AJ. Precursor cells of mechanocytes. Int Rev Cytol. 1976. 47:327–359.
Article7. Friedenstein AJ, Chailakhyan RK, Gerasimov UV. Bone marrow osteogenic stem cells: in vitro cultivation and transplantation in diffusion chambers. Cell Tissue Kinet. 1987. 20:263–272.
Article8. Friedenstein AJ, Petrakova KV, Kurolesova AI, Frolova GP. Heterotopic of bone marrow. Analysis of precursor cells for osteogenic and hematopoietic tissues. Transplantation. 1968. 6:230–247.9. Owen M, Friedenstein AJ. Stromal stem cells: marrow-derived osteogenic precursors. Ciba Found Symp. 1988. 136:42–60.
Article10. Campagnoli C, Roberts IA, Kumar S, Bennett PR, Bellantuono I, Fisk NM. Identification of mesenchymal stem/progenitor cells in human first-trimester fetal blood, liver, and bone marrow. Blood. 2001. 98:2396–2402.
Article11. De Ugarte DA, Morizono K, Elbarbary A, Alfonso Z, Zuk PA, Zhu M, et al. Comparison of multi-lineage cells from human adipose tissue and bone marrow. Cells Tissues Organs. 2003. 174:101–109.
Article12. Erices A, Conget P, Minguell JJ. Mesenchymal progenitor cells in human umbilical cord blood. Br J Haematol. 2000. 109:235–242.
Article13. Jiang Y, Vaessen B, Lenvik T, Blackstad M, Reyes M, Verfaillie CM. Multipotent progenitor cells can be isolated from postnatal murine bone marrow, muscle, and brain. Exp Hematol. 2002. 30:896–904.
Article14. Kuznetsov SA, Mankani MH, Gronthos S, Satomura K, Bianco P, Robey PG. Circulating skeletal stem cells. J Cell Biol. 2001. 153:1133–1140.
Article15. Lee MW, Yang MS, Park JS, Kim HC, Kim YJ, Choi J. Isolation of mesenchymal stem cells from cryopreserved human umbilical cord blood. Int J Hematol. 2005. 81:126–130.
Article16. Noort WA, Kruisselbrink AB, in't Anker PS, Kruger M, van Bezooijen RL, de Paus RA, et al. Mesenchymal stem cells promote engraftment of human umbilical cord blood-derived CD34(+) cells in NOD/SCID mice. Exp Hematol. 2002. 30:870–878.
Article17. Young HE, Steele TA, Bray RA, Hudson J, Floyd JA, Hawkins K, et al. Human reserve pluripotent mesenchymal stem cells are present in the connective tissues of skeletal muscle and dermis derived from fetal, adult, and geriatric donors. Anat Rec. 2001. 264:51–62.
Article18. Zvaifler NJ, Marinova-Mutafchieva L, Adams G, Edwards CJ, Moss J, Burger JA, et al. Mesenchymal precursor cells in the blood of normal individuals. Arthritis Res. 2000. 2:477–488.
Article19. Rebelatto CK, Aguiar AM, Moretão MP, Senegaglia AC, Hansen P, Barchiki F, et al. Dissimilar differentiation of mesenchymal stem cells from bone marrow, umbilical cord blood, and adipose tissue. Exp Biol Med (Maywood). 2008. 233:901–913.
Article20. Sakaguchi Y, Sekiya I, Yagishita K, Muneta T. Comparison of human stem cells derived from various mesenchymal tissues: superiority of synovium as a cell source. Arthritis Rheum. 2005. 52:2521–2529.
Article21. Zuk PA, Zhu M, Mizuno H, Huang J, Futrell JW, Katz AJ, et al. Multilineage cells from human adipose tissue: implications for cell-based therapies. Tissue Eng. 2001. 7:211–228.
Article22. Strem BM, Hicok KC, Zhu M, Wulur I, Alfonso Z, Schreiber RE, et al. Multipotential differentiation of adipose tissue-derived stem cells. Keio J Med. 2005. 54:132–141.
Article23. Hoffmann J, Glassford AJ, Doyle TC, Robbins RC, Schrepfer S, Pelletier MP. Angiogenic effects despite limited cell survival of bone marrow-derived mesenchymal stem cells under ischemia. Thorac Cardiovasc Surg. 2010. 58:136–142.
Article24. Mylotte LA, Duffy AM, Murphy M, O'Brien T, Samali A, Barry F, et al. Metabolic flexibility permits mesenchymal stem cell survival in an ischemic environment. Stem Cells. 2008. 26:1325–1336.
Article25. Matsumoto D, Shigeura T, Sato K, Inoue K, Suga H, Kato H, et al. Influences of preservation at various temperatures on liposuction aspirates. Plast Reconstr Surg. 2007. 120:1510–1517.
Article26. Keck M, Zeyda M, Gollinger K, Burjak S, Kamolz LP, Frey M, et al. Local anesthetics have a major impact on viability of preadipocytes and their differentiation into adipocytes. Plast Reconstr Surg. 2010. 126:1500–1505.
Article27. Preece A. A manual for histologic technicians. 1972. Boston: Little, Brown.28. Hu Y, Liao L, Wang Q, Ma L, Ma G, Jiang X, et al. Isolation and identification of mesenchymal stem cells from human fetal pancreas. J Lab Clin Med. 2003. 141:342–349.
Article29. Martin JY, Dean DD, Cochran DL, Simpson J, Boyan BD, Schwartz Z. Proliferation, differentiation, and protein synthesis of human osteoblast-like cells (MG63) cultured on previously used titanium surfaces. Clin Oral Implants Res. 1996. 7:27–37.
Article30. Woodbury D, Schwarz EJ, Prockop DJ, Black IB. Adult rat and human bone marrow stromal cells differentiate into neurons. J Neurosci Res. 2000. 61:364–370.
Article31. Santa María L, Rojas CV, Minguell JJ. Signals from damaged but not undamaged skeletal muscle induce myogenic differentiation of rat bone-marrow-derived mesenchymal stem cells. Exp Cell Res. 2004. 300:418–426.
Article32. Xu W, Zhang X, Qian H, Zhu W, Sun X, Hu J, et al. Mesenchymal stem cells from adult human bone marrow differentiate into a cardiomyocyte phenotype in vitro. Exp Biol Med (Maywood). 2004. 229:623–631.
Article33. Eom YW, Lee JE, Yang MS, Jang IK, Kim HE, Lee DH, et al. Effective myotube formation in human adipose tissue-derived stem cells expressing dystrophin and myosin heavy chain by cellular fusion with mouse C2C12 myoblasts. Biochem Biophys Res Commun. 2011. 408:167–173.
Article34. Yoo KH, Jang IK, Lee MW, Kim HE, Yang MS, Eom Y, et al. Comparison of immunomodulatory properties of mesenchymal stem cells derived from adult human tissues. Cell Immunol. 2009. 259:150–156.
Article35. Gronthos S, Franklin DM, Leddy HA, Robey PG, Storms RW, Gimble JM. Surface protein characterization of human adipose tissue-derived stromal cells. J Cell Physiol. 2001. 189:54–63.
Article36. Zuk PA, Zhu M, Ashjian P, De Ugarte DA, Huang JI, Mizuno H, et al. Human adipose tissue is a source of multipotent stem cells. Mol Biol Cell. 2002. 13:4279–4295.
Article37. Puissant B, Barreau C, Bourin P, Clavel C, Corre J, Bousquet C, et al. Immunomodulatory effect of human adipose tissue-derived adult stem cells: comparison with bone marrow mesenchymal stem cells. Br J Haematol. 2005. 129:118–129.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Analysis of Stromal Vascular Fraction from Lipoaspirates: Our Institute's Experiences
- Adipose Tissue Derived Mesenchymal Stem Cells
- Concise Review: Differentiation of Human Adult Stem Cells Into Hepatocyte-like Cells In vitro
- Adipose Tissue - Adequate, Accessible Regenerative Material
- Adipose-derived stem cells: characterization and clinical application